From left to right: James Cahoon and Taylor Teitsworth. Credit: Steve Exum
Researchers at the University of North Carolina at Chapel Hill Department of Chemistry have engineered silicon nanowires that can convert sunlight into electricity by splitting water into oxygen and hydrogen gas, a greener alternative to fossil fuels.
Fifty years ago, scientists first demonstrated that liquid water can be split into oxygen and hydrogen gas using electricity produced by illuminating a semiconductor electrode. Although hydrogen generated using solar power is a promising form of clean energy, low efficiencies and high costs have hindered the introduction of commercial solar-powered hydrogen plants.
An economic feasibility analysis suggests that using a slurry of electrodes made from nanoparticles instead of a rigid solar panel design could substantially lower costs, making solar-produced hydrogen competitive with fossil fuels. However, most existing particle-based light-activated catalysts, also referred to as photocatalysts, can absorb only ultraviolet radiation, limiting their energy-conversion efficiency under solar illumination.
James Cahoon, Ph.D., Hyde Family Foundation Professor of Chemistry in UNC-Chapel Hill's College of Arts and Sciences, and his colleagues in the department have been working on the chemical synthesis of semiconductor nanomaterials with unique physical properties that can enable a range of technologies, from solar cells to solid-state memory. Cahoon serves as the corresponding author of the findings published Feb. 9 in Nature.
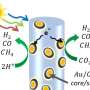
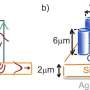
Cahoon and his team designed new silicon nanowires to have multiple solar cells along their axis so that they could produce the power needed to split water.
"This design is unprecedented in previous reactor designs and allows silicon to be used for the first time in a PSR," explained Taylor Teitsworth, a postdoctoral research associate in Cahoon's lab.
Silicon absorbs both visible and infrared light. It has historically been a top choice for solar cells, also referred to as photovoltaic cells and semiconductors, owing to this and other properties—including its abundance, low toxicity and stability. With its electronic properties, the only way to drive water splitting wirelessly with silicon particles is to encode multiple photovoltaic cells in each particle. This can be achieved by generating particles that contain multiple interfaces, called junctions, between two different forms of silicon—p-type and n-type semiconductors.
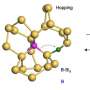
Previously, Cahoon's research focused on a bottom-up synthesis and spatially controlled modulation of silicone with boron for p-type nanowires and with phosphorus for n-type nanowires to impart desirable geometries and functionalities.
"We used this approach to create a new class of water-splitting multijunction nanoparticles. These combine the material and economic advantages of silicon with the photonic advantages of nanowires that have a diameter smaller than the wavelength of absorbed light," said Cahoon. "Owing to the inherent asymmetry of the wire junctions, we were able to use a light-driven electrochemical method to deposit the co-catalysts selectively onto the ends of the wires to enable water splitting."
More information: Taylor S. Teitsworth et al, Water splitting with silicon p–i–n superlattices suspended in solution, Nature (2023). DOI: 10.1038/s41586-022-05549-5
Designer silicon nanowires produce hydrogen from water and light, Nature (2023). DOI: 10.1038/d41586-023-00154-6
Journal information: Nature
Provided by University of North Carolina at Chapel Hill