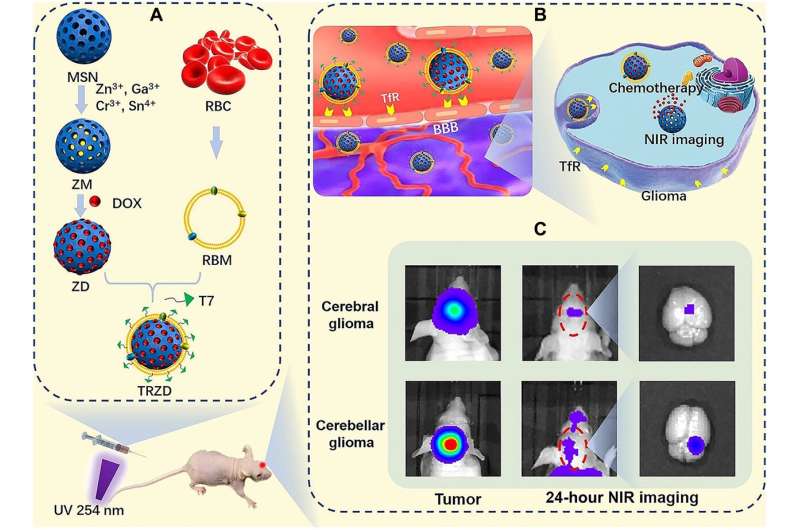
Scheme of the formation of TRZD and the proposed mechanism of action for the diagnosis and treatment of cerebral and cerebellar gliomas. (A) Design of TRZD. ZGOCS was loaded in mesoporous silica NPs (MSNs) to form ZM (ZGOCS@MSN). DOX was loaded in ZM to form ZD. RBM was extracted from red blood cell (RBC) and coated on ZD to form RZD (DOX-ZGOCS@MSN@RBM). T7 peptide, for both BBTB penetration and glioma targeting, was conjugated on RZD to form TRZD (DOX-ZGOCS@MSN@RBM-T7). The suspension of TRZD was excited by 254-nm UV light for 5 min and then injected through the tail vein into the mice. (B) Blood-brain barrier (BBB) penetration and glioma targeting of TRZD. In the brain, TRZD penetrates through the BBB via receptor-mediated transcytosis, because T7 exhibits high binding affinity to the TfR, which is overexpressed on BBB. TRZD will also target at glioma cells since TfR is also overexpressed on tumor cells, including glioma cells. After the receptor-mediated endocytosis to glioma cells, DOX will perform a controlled release from TRZD for the chemotherapy of glioma. (C) NIR imaging of cerebral and cerebellar gliomas. TRZD, which contains ZGOCS, will show excellent rechargeable NIR PL for more than 30 hours in vivo with good tissue penetration, which enables long-term autofluorescence-free imaging of orthotopic glioma at both cerebrum and cerebellum. Credit: Science Advances (2022). DOI: 10.1126/sciadv.abm7077
A Hong Kong Baptist University (HKBU) collaborative research team has synthesized a nanoparticle named TRZD that can perform the dual function of diagnosing and treating glioma in the brain. It emits persistent luminescence for the diagnostic imaging of glioma tissues in vivo and inhibits the growth of tumor cells by aiding the targeted delivery of chemotherapy drugs.
The nanoparticle offers hope for the early diagnosis and treatment of glioma, especially cerebellar glioma, which is even harder to detect and cure with existing methods. The research results have been published in the journal Science Advances.
Limitations of existing diagnostic and therapeutic approaches
Glioma is the most common form of malignant primary brain tumor, and it accounts for about one-third of all brain tumors. Magnetic resonance imaging (MRI) is commonly used to diagnose glioma, but the technology is not that sensitive. Cerebellar glioma, a relatively rare brain tumor, is even harder to detect with MRI. To facilitate early detection and treatment, an alternative method with improved sensitivity and precision is needed to diagnose glioma.
Doxorubicin, a chemotherapy agent, is an effective treatment for glioma. However, its application may also damage normal cells, and it is associated with a range of side effects. To enhance doxorubicin's clinical efficacy and minimize its side effects, a novel approach is needed to apply the drug to tumor cells in a more targeted manner.
In response to the diagnostic and therapeutic needs of glioma, a research team co-led by Dr. Wang Yi, Assistant Professor of the Department of Chemistry at HKBU, and Professor Law Ga-lai, Professor of the Department of Applied Biology and Chemical Technology at the Hong Kong Polytechnic University, has synthesized a novel near-infrared (NIR) persistent luminescence nanoparticle called TRZD, which can play a dual role in diagnostic imaging and as a drug carrier for glioma.
TRZD has the characteristic of emitting NIR persistent luminescence after excitation with ultraviolet (UV) light. The basic structure of TRZD is a combination of nanoparticles, loaded with the mesoporous structure of silica, which makes it a good carrier of doxorubicin particles. Its surface is coated with red blood cell membranes to increase its stability, and it is embedded with T7 peptides. T7 peptides have a strong affinity for transferrin receptors which are abundant on the surface of tumor cells, and they can facilitate TRZD's penetration through the blood-brain barrier.
An imaging probe for glioma diagnosis
The research team evaluated the efficacy of TRZ (i.e. TRZD without doxorubicin) in diagnostic imaging for glioma with a mouse model. TRZ particles were first excited by UV light to initiate luminescence. Mice with tumor tissues injected into their cerebrum and cerebellum were then treated with TRZ. In the following 24 hours, TRZ luminescence was detected at the tumor sites of the mice.
However, when the same experiment was conducted with TRZ without T7 peptides, and TRZ without both the red blood cell membrane coating and T7 peptides, no luminescence was detected at the tumor sites of the mice. The results show that the red blood cell membrane coating can prolong the function of TRZ by stabilizing the nanoparticle, and it can slow down its natural uptake by the human body. On the other hand, T7 peptides are instrumental in TRZ's penetration into and accumulation in tumor cells, so that it can perform its imaging function for glioma.
Dr. Wang said, "Our experiment suggests that TRZ is a promising bioimaging agent for the diagnosis of glioma. It was observed that TRZ's luminescence can be detected in tumor cells in both the cerebrum and cerebellum regions of the brain, which is an encouraging result because glioma in the cerebellum region is difficult to detect with existing diagnostic methods. As a result, TRZ offers new hope for the timely and accurate diagnosis of glioma."
TRZD inhibits the growth of glioma and extends the lifespan of mice
The research team further evaluated the anti-tumor efficacy of TRZD using a group of mice who had had their cerebrum and cerebellum injected with tumor tissues. After applying TRZD for 15 days, the average diameter of their tumors was reduced to 1 mm. They also survived 20 days longer on average compared to the control group, who had not received TRZD. Besides, cell death was observed in the tumor region but not in normal brain tissue.
Dr. Wang said, "The experimental results indicate that TRZD's therapeutic effect on glioma has good selectivity, because doxorubicin is brought specifically to tumor cells due to T7 peptide's strong affinity with tumor cells' surface receptors and its ability to penetrate the blood-brain barrier. As a result, doxorubicin can be applied in a more targeted manner, and hopefully its side effects can be minimized with a reduced drug dosage.
"We concluded that TRZD demonstrates promising potential, and it could be developed into a new generation of anti-glioma drugs that can perform the dual function of diagnosis and treatment. It also offers hope for the development of treatment protocols for other brain diseases."
More information: Jianglong Kong et al, Biomimetic multifunctional persistent luminescence nanoprobes for long-term near-infrared imaging and therapy of cerebral and cerebellar gliomas, Science Advances (2022). DOI: 10.1126/sciadv.abm7077
Journal information: Science Advances
Provided by Hong Kong Baptist University