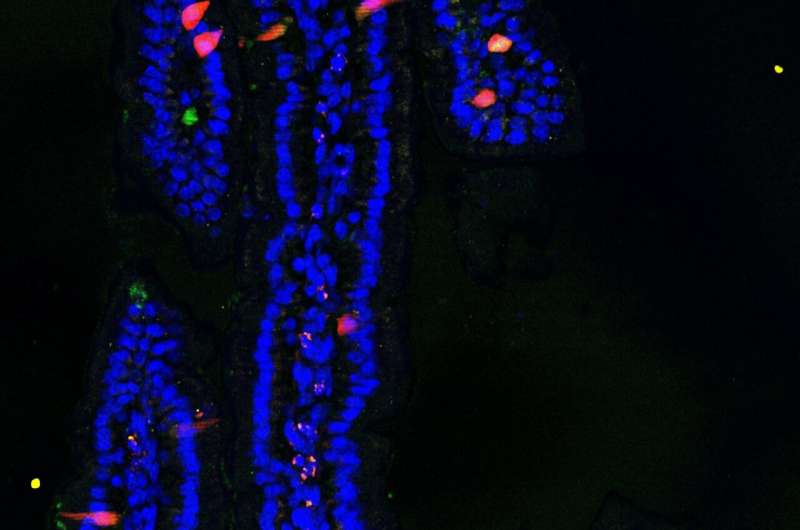
Section of the mouse small intestine in which cells that produce different hormones are labeled with different colors. Credit: Joep Beumer, © Hubrecht Institute
Researchers from the group of Hans Clevers at the Hubrecht Institute (KNAW) in the Netherlands and their collaborators shed new light on the origin and function of hormone-producing cells in the intestine and open new avenues to tweak gut hormone production to treat human disease. Their results were recently published in Nature Cell Biology and in Cell.
Did you ever wonder where that sudden feeling of hunger comes from when your empty stomach rumbles? This happens when thousands of nutrient-sensitive cells, or enteroendocrine cells, scattered throughout the stomach and intestine release millions of tiny vesicles filled with the hunger hormone ghrelin into the bloodstream. Such hormones act as the gut's primary method of communication and coordination with more distant parts of the digestive tract or other organs such as the pancreas and the brain.
In response to certain stimuli, different enteroendocrine cells produce different hormones, which induce hunger or satiety, coordinate movement of intestinal muscles, stimulate the repair of the intestine's protective cell layer or promote a higher output of insulin from the pancreas. The latter is especially interesting in patients with type II diabetes, which, on their own, are unable to produce sufficient insulin to stabilize their glucose levels. One of the most successful treatments for diabetes is based on the gut hormone GLP1, with which such patients are able to control their blood glucose without the need of insulin injections.
Less than 1 percent of the cells in the intestinal lining are enteroendocrine cells. This 1 percent is again split into many subtypes that produce different hormones. Therefore, a specific type of enteroendocrine cell is hard to find. It's like looking for a few diamonds, rubies, and emeralds in a truck full of pebbles. You can weigh the load, measure it, grind it down and analyze the mineral composition, but this will tell you a lot more about the pebbles than about the gemstones.
To study these rare cells, the researchers combined single-cell sequencing with a method to determine the age of each cell. To stay with the gemstone analogy, they made all gemstones shine so brightly that they could be picked out from the pile and analyzed individually. In addition, the color of the gemstones told the researchers each cell's age. As a result, they could study the development of enteroendocrine cells.
Intestinal crypts, the birthplace of intestinal cells, in the small intestine of a mouse. Labeled in green and red are the enteroendocrine cells. Younger cells are more green, older cells are more red. Credit: Anne Rios and Helmuth Gehart, © Princess Máxima Center and Hubrecht Institute.
Tweaking the system
Enteroendocrine cells are continuously produced in the intestines and live for several weeks. Surprisingly, the researchers found that many enteroendocrine cells change their hormone production while they were aging. This ability of a cell to switch hormone production and thus function is highly interesting in the context of therapy. Once researchers understand the signals that control it, they may be able to stimulate the intestine to increase the production of specific hormones to treat diabetes, obesity or inflammatory bowel disease. The researchers already showed that manipulation of one of these signals could change hormone levels, including those of GLP1, in mice.
Single-cell sequencing is a relatively new technology named as "Breakthrough of the year 2018" by the scientific journal Science. Using this technique, researchers can read the activity of genes at the resolution of individual cells. This tells them what genetic program was active in a cell, and by derivation, what its identity is (for instance, a skin cell or an immune cell).
Researchers were already able to read gene activity in a tissue, but these readouts were always done on many thousands of cells pooled together. Now, with single-cell sequencing, researchers are able to read the gene activity of every individual cell. This improvement is comparable to the difference between a classical geographical map, in which a city is represented as one patch of color, and Google Maps, which can zoom down to every house individually, finding interesting details in the process.
Since the technique is widely applicable in research, many scientists are now using it to analyze their organ or disease of interest at single-cell resolution.
However, performing single-cell sequencing and interpreting its results requires highly specialized lab equipment and data analysis algorithms. To solve this issue, a startup called Single Cell Discoveries was created at the Hubrecht Insitute that performs single-cell sequencing as a service to researchers and clinical institutions around the world.
More information: Joep Beumer et al. Enteroendocrine cells switch hormone expression along the crypt-to-villus BMP signalling gradient. Nature Cell Biology, 2018. www.nature.com/articles/s41556-018-0143-y
Helmuth Gehart et al. Identification of enteroendocrine regulators by real-time single-cell differentiation mapping. Cell, 2019. DOI: 10.1016/j.cell.2018.12.029 , www.cell.com/cell/fulltext/S0092-8674(18)31643-X
Journal information: Nature Cell Biology , Cell , Science
Provided by Hubrecht Institute