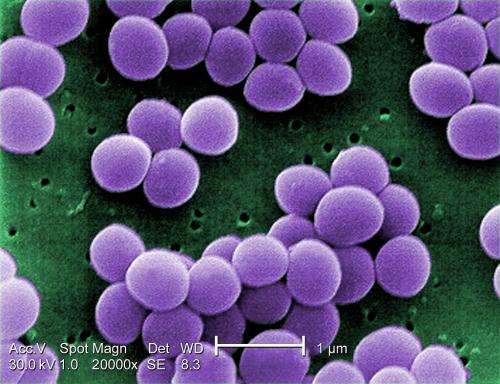
Scanning electron micrograph of S. aureus; false color added. Credit: CDC
(Phys.org)—A large team of researchers from several institutions in the U.S. and one in Denmark, working for Genentech, has found that binding an antibiotic to an antibody can be more effective in treating bacterial infections than current methods. In their paper published in the journal Nature, the group describes how their procedure works and the results they found when testing it in mice. Wolf-Dietrich Hardt with the Institute of Microbiology, ETH in Switzerland offers a News & Views piece on the work done by the team and suggests that if the technique proves to work the same way in humans, it could lead to the development of drugs meant to kill infections that are less harmful to good bacteria in the body.
Prior research has found that one of the ways that bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) become resistant to drugs meant to kill them, is by hiding out in host cells, where antibiotics cannot find them. Many in the field believe this is why some bacterial infections return so quickly after antibiotics are stopped. In this new effort, the researchers developed a way to cause a common antibiotic to bind to an antibody until it gets inside a host cell harboring a certain type of bacteria—then, the bond is broken and the antibiotic kills the bacteria.
More specifically, they created an anti-S. aureus antibody that grabs onto one type of sugar found on the exterior part of the bacteria. Next, they attached an antibiotic called rifalogue via a linker molecule to cysteines on the antibody. The result was the creation of antibody-antibiotic conjugates, or AACs. Once they make their way inside a certain host cell, the linker molecule is removed releasing the antibiotic to do its job.
The team reports that injecting the AACs they created into infected mice was far more effective at completely wiping out an infection than standard treatments used today. Hardt notes that if the technique proves to be successful in humans, the use of AACs could also mean less development of resistance in bacterial strains because the technique allows for focusing on just one type of bacteria.
More information: Sophie M. Lehar et al. Novel antibody–antibiotic conjugate eliminates intracellular S. aureus, Nature (2015). DOI: 10.1038/nature16057
Abstract
Staphylococcus aureus is considered to be an extracellular pathogen. However, survival of S. aureus within host cells may provide a reservoir relatively protected from antibiotics, thus enabling long-term colonization of the host and explaining clinical failures and relapses after antibiotic therapy. Here we confirm that intracellular reservoirs of S. aureus in mice comprise a virulent subset of bacteria that can establish infection even in the presence of vancomycin, and we introduce a novel therapeutic that effectively kills intracellular S. aureus. This antibody–antibiotic conjugate consists of an anti-S. aureus antibody conjugated to a highly efficacious antibiotic that is activated only after it is released in the proteolytic environment of the phagolysosome. The antibody–antibiotic conjugate is superior to vancomycin for treatment of bacteraemia and provides direct evidence that intracellular S. aureus represents an important component of invasive infections.
Journal information: Nature
© 2015 Phys.org