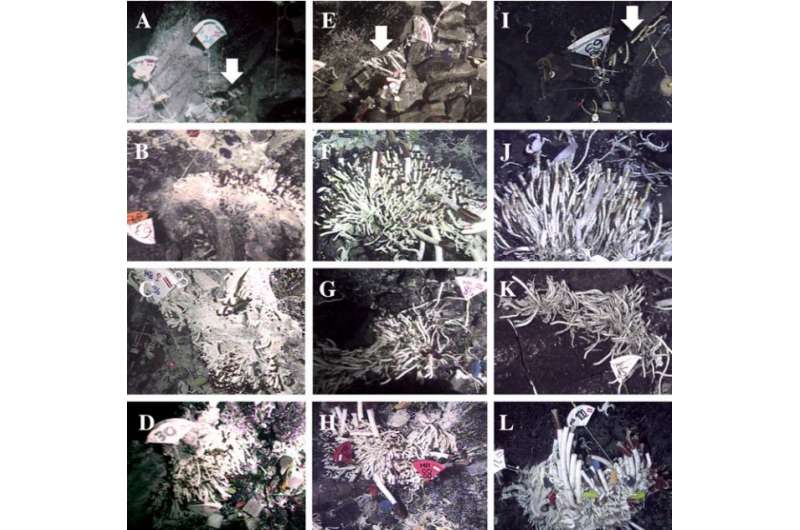
Temporal colonization of vent patches over 4 y after a volcanic eruption in 2005/2006. Patches of T. jerichonana at Sketchy. Credit: PNAS 2015 ; published ahead of print August 17, 2015, doi:10.1073/pnas.1501160112
(Phys.org)—Deep within oceanic hydrothermal vents, the thiotrophic gamma-proteobacterial endosymbiont Candidatus Endoriftia lives in happy symbiosis with its host organism, the sessile tubeworm Riftia pachyptila: The tubeworm provides all the substrates the endosymbiont needs for chemosynthesis and, in return, Endoriftia releases fixed organic carbon for the tubeworm host, which is gutless, and would otherwise have no source of nutrition.
In order to propagate and persist, Endoriftia needs to transmit to other tubeworm individuals, but until recently, researchers were unsure how this was achieved, as Riftia has no body openings. This mode of intergenerational persistence is called horizontal transmission, as opposed to vertical transmission, in which hosts transmit endosymbionts directly to their offspring.
A group of researchers recently explored Endoriftia transmission experimentally to determine how and when symbionts were released into the unique environment of hydrothermal vents, and what surfaces it occupies until populating a clump of new, aposymbiotic tubeworms. They have published their results in the Proceedings of the National Academy of Sciences.
The researchers hypothesize that Endoriftia escapes the tissues of the newly dead tubeworms into the surrounding environment. To discover the mode of transmission, the researchers simulated the high-pressure, high-temperature conditions of a hydrothermal vent in a laboratory setting.
To collect tubeworm samples, the researchers embarked on four cruises that included excursions in a submersible. They deployed colonization devices at several sites and collected them once they were populated with specimens. Live worms were dissected and samples placed in specially designed capsules, which were incubated in sterile, filtered seawater in high-pressure flowthrough vessels.
All samples were analyzed via fluorescence in situ hybridization (FISH) analysis. The results revealed that Endoriftia rapidly abandons dead host tissue in very large numbers and recruits to such surfaces found in hydrothermal vent conditions.
While populating the tubeworms, Endoriftia lives inside cells called bacteriocytes. Though the researchers found free Endoriftia in the seawater, they found no evidence of bacteriocytes, a strong indication that Endoriftia makes an active escape from the bacteriocyte following the death of the host. They note that while the endosymbiont does not express flagella while associated with the tubeworm, they do produce flagella while independent of a host.
Further, the researchers explored the degradation processes by evaluating the integrity of the host and symbiont membranes. They found that under deep-sea conditions, most of the symbiont and bacteriocyte membranes remained intact for about 10 days. They note, however, that "even after six days of vent incubation, a few morphologically intact symbionts were detected, but in most symbionts, membranes were distorted." This suggests a rough timeframe for the repopulation of free-living symbionts.
Thus, the study uniquely demonstrates Endoriftia escaping from dead tubeworm hosts and seeding the surrounding environment in the singularly dynamic and fluctuating deep-sea hydrothermal environment. The researchers conclude, "Now that this principal mode of host-associated and free-living symbiont connectivity is known, we can start to decipher the stability of this mutualism in situ in the framework of population genetics and metapopulation ecology."
More information: "Endosymbionts escape dead hydrothermal vent tubeworms to enrich the free-living population." PNAS 2015 ; published ahead of print August 17, 2015, DOI: 10.1073/pnas.1501160112
Abstract
Theory predicts that horizontal acquisition of symbionts by plants and animals must be coupled to release and limited dispersal of symbionts for intergenerational persistence of mutualisms. For deep-sea hydrothermal vent tubeworms (Vestimentifera, Siboglinidae), it has been demonstrated that a few symbiotic bacteria infect aposymbiotic host larvae and grow in a newly formed organ, the trophosome. However, whether viable symbionts can be released to augment environmental populations has been doubtful, because (i) the adult worms lack obvious openings and (ii) the vast majority of symbionts has been regarded as terminally differentiated. Here we show experimentally that symbionts rapidly escape their hosts upon death and recruit to surfaces where they proliferate. Estimating symbiont release from our experiments taken together with well-known tubeworm density ranges, we suggest a few million to 1.5 billion symbionts seeding the environment upon death of a tubeworm clump. In situ observations show that such clumps have rapid turnover, suggesting that release of large numbers of symbionts may ensure effective dispersal to new sites followed by active larval colonization. Moreover, release of symbionts might enable adaptations that evolve within host individuals to spread within host populations and possibly to new environments.
Journal information: Proceedings of the National Academy of Sciences
© 2015 Phys.org