Brain sensors and electronic tags that dissolve. Boosting the potential of renewable energy sources. These are examples of the latest research from two pioneering scientists selected as this year's Kavli lecturers at the 247th National Meeting & Exposition of the American Chemical Society (ACS).
The meeting features more than 10,000 presentations from the frontiers of chemical research, and is being held here through Thursday. Two of these talks are supported by The Kavli Foundation, a philanthropic organization that encourages basic scientific innovation. These lectures, which are a highlight of the conference, shine a spotlight on the work of both young and established researchers who are pushing the boundaries of science to address some of the world's most pressing problems.
Tackling health and sustainability issues simultaneously, John Rogers, Ph.D., is developing a vast toolbox of materials—from magnesium and silicon to silk and even rice paper—to make biodegradable electronics that can potentially be used in a range of applications. He will deliver "The Fred Kavli Innovations in Chemistry Lecture."
"What we're finding is that there's a robust and diverse palette of material options at every level," said Rogers, who's with the University of Illinois, Urbana-Champaign. "For the conductor, for the semiconductor, for the insulating layer and the package and the substrate, one can pick and choose materials depending on the application's requirements."
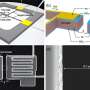
Rogers' team is working to incorporate some of these elements in sensors that can, for example, detect the early onset of swelling and temperature changes in the brain after head injuries and then vanish when they're no longer needed. Today, devices designed for these purposes are wired—they have to be implanted and later completely removed once they're no longer needed. Rogers' sensor could be implanted but work wirelessly and, after use, "simply disappear." That eliminates the risk of infection and other complications associated with having to remove devices surgically. Rogers has successfully tested early prototypes of sensors in laboratory animals and envisions that such devices could be used one day in human patients.
His group is also working on biodegradable radio-frequency identification tags, or RFID tags. Currently, RFIDs are produced by the billions and used in everything from jeans for accurately tracking inventory to smart cards and injected into pets. They are also found in product packaging that ends up in landfills. Using cellulose, zinc and silicon, Rogers has successfully made dissolvable RFID tags in the lab. The next step would be figuring out how to scale production up and commercialize it.
"We're quite optimistic," Rogers said. "We see the way forward and are about halfway there."
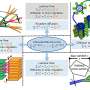
Delivering the "The Kavli Foundation Emerging Leader in Chemistry Lecture" is Emily Weiss, Ph.D., of Northwestern University. Her lab is focused on getting the most power possible out of mixed and matched nanomaterials that are being developed to maximize renewable energy sources. Scientists can now engineer these materials with unprecedented precision to capture large amounts of energy—for example, from the sun and heat sources. But getting all that energy from these materials and pushing it out into the world to power up homes and gadgets have been major obstacles.
"Electric current originates from the movement of electrons through a material," Weiss explained. "But as they move through a material or device, they encounter places where they have to jump from one type of material to another at what's called an interface. By interfaces, I mean places where portions of the material that are not exactly alike meet up. The problem is when an electron has to cross from one material to another, it loses energy."
As structures in materials get smaller, the interface problem becomes amplified because nanomaterials have more surface area compared to their volume. So electrons in these advanced devices have to travel across more and more interfaces, and they lose energy as heat every time.
But thanks to the latest advances in analytical instruments and computing power, Weiss' group is poised to turn this disadvantage into a plus. "Rather than seeing all these interfaces as a negative, now we don't need to consider it a drawback," she said. "We can design an interface such that we can get rid of defects and get rid of this slowdown. We can actually use carefully designed interfaces to enhance the properties of your device. That sort of philosophy is starting to take hold."
More information: 1. Biodegradable electronics, John Rogers, Ph.D.:
Abstract
A remarkable feature of the modern integrated circuit is its ability to operate in a stable fashion, with almost perfect reliability. Recently developed classes of electronic materials create an opportunity to engineer the opposite outcome, in the form of devices that dissolve completely in water, with harmless end products. The enabled applications range from 'green' consumer electronics to bio-resorbable medical implants – none of which would be possible with technologies that exist today. This talk summarizes recent work on this physically 'transient' type of electronics, from basic advances in materials chemistry, to fundamental studies of dissolution reactions, to engineering development of complete sets of device components, sensors and integrated systems. An 'electroceutical' bacteriocide designed for treatment of surgical site infections provides an application example.
2. Behavior of electrons at nanoscopic organic/inorganic interfaces, Emily Weiss, Ph.D.:
Abstract
The behavior of electrons and energy at interfaces between different types or phases of materials is an active research area of both fundamental and technological importance. Such interfaces often result in sharp free energy gradients that provide the thermodynamic driving force for some of the most crucial processes for energy conversion: migration of energy and charge carriers, conversion of excited states to mobile charge carriers, and redox-driven chemical reactions. Nanostructured materials are defined by high surface area-to-volume ratios, and should therefore be ideal for the job of energy conversion; however, they have a structural and chemical complexity that does not exist in bulk materials, and which presents a formidable challenge: mitigate or eliminate energy barriers to electron and energy flux that inevitably result from forcing dissimilar materials to meet in a spatial region of atomic dimensions. Chemical functionalization of nanostructured materials is perhaps the most versatile and powerful strategy for controlling the potential energy landscape of their interfaces, and for minimizing losses in energy conversion efficiency due to interfacial structural and electronic defects. Using metal and semiconductor nanoparticles as model systems, this talk will explore the power of tuning the chemistry at the organic-inorganic interface within colloidal semiconductor and metal nanoparticles as a strategy for controlling their structure and properties.