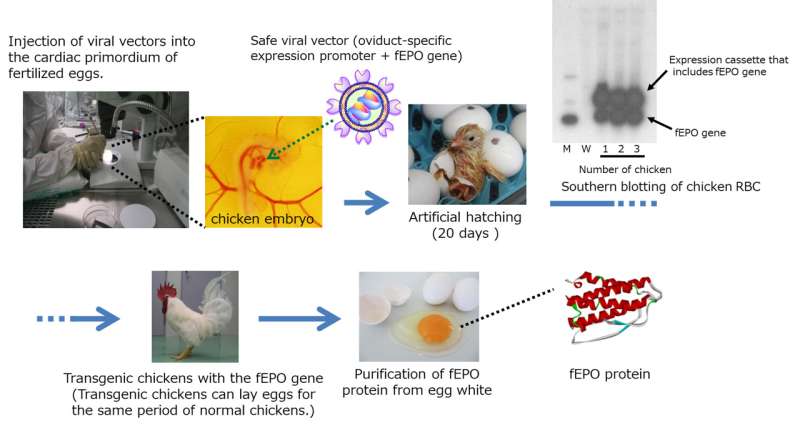
Fig.1 The production of fEPO by transgenic chicken. Credit: Kaneka
Many cats develop chronic renal disorders as they age. As chronic renal disorders progress, the secretion of erythropoietin (EPO), a hematopoietic factor produced in the kidneys, is decreased, which causes renal anemia. Veterinary medicine, until now, has only had an option of using erythropoietin (human EPO) derived from human amino acid sequence to treat severe renal anemia. However, since human EPO is a heterologous protein for cats, there has been an issue of adverse reactions such as allergic reactions. Therefore, there has been a great demand for a renal anemia remedy with a low level of adverse reactions for cats, that can lower the burden on both cats and their owners.
In the JST Adaptable and Seamless Technology Transfer Program through Target-driven R&D (A-STEP), corporative development for an "anemia remedy produced through transgenic chicken technology" has been entrusted to Kaneka Corporation, based on the research results of Professor Shinji Iijima, currently of Aichi Institute of Technology (Nagoya University at the time of development) and others.
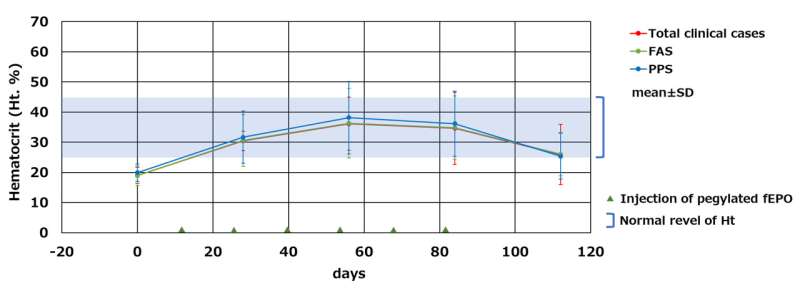
In this development, transgenic chicken production technology was successfully used to develop feline (cat) erythropoietin (fEPO) with feline-derived amino acid sequences. A viral vector expressing cat EPO genes was injected into the embryo of fertilized chicken egg by micro injection, by which fEPO was produced within the egg albumin fraction. An established process in which polyethylene glycol (PEG) modification was conducted on fEPO collected and refined from chicken eggs lead to produce Pegylated fEPO with a longer efficacy period compared to unmodified fEPO (Fig.1). The Pegylated fEPO was tested on 60 cases in clinical trial, and its effectiveness and safety were evaluated (Fig.2). JST certified the results of the development related to this issue to be a success.
Fig.2 Changes in hematocrit levels after administration of fEPOFull analysis set (FAS) : All subjects which fEPO was administered one or more times.Per Protocol Set (PPS) : All subjects in the FAS which completed administration of cat EPO in treatment period. Credit: Kaneka
The developed Pegylated fEPO has little adverse reactions, therefore, serial administration to cats from the early stages in which symptoms of anemia occur is possible. Moreover, amelioration of symptoms such as loss of appetite and vitality due to anemia promises to lower the burden and improve the QOL of both the cat and its owner.
Kaneka will license out the developed outcome to domestic animal drug companies, and the companies assigned for the license will manufacture and sell the product.
A-STEP is a technology transfer support program whose aim is to put the research results by public research institutes into practical applications as important technology in the national economy, and thus to give some of their profit back to society. For detailed information, see www.jst.go.jp/tt/EN/univ-ip/a-step.html
Provided by Japan Science and Technology Agency (JST)