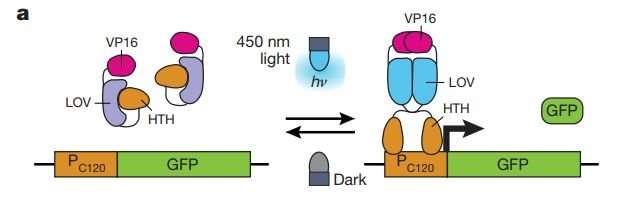
Reversible OptoEXP system based on VP16–EL222 that is sensitive to 450 nm light. hν indicates the energy of photons. HTH, helix–turn–helix DNA-binding domain. Credit: Nature (2018). DOI: 10.1038/nature26141
A team of researchers at Princeton University has developed a way to cause yeast to produce more isobutanol, a possible candidate for use as a biofuel. In their paper published in the journal Nature, the team describes their use of optogenetics to increase isobutanol production by yeast.
As the search for alternatives to petroleum continues, scientist continue to look for new sources of biofuels. In this new effort, the researchers genetically altered yeast to produce more isobutanol in the absence of light.
As the researchers note, it would be convenient if yeast could produce isobutanol all the time, but that does not appear to be possible, because isobutanol is actually toxic to yeast—if it builds up, the yeast will die. Because of that, yeast must be allowed to go into a growth phase in which the yeast builds up precursor substances that it needs to remain healthy. Prior efforts have shown that it is possible to get yeast to switch between production and growth phases, but such efforts have not been uniform, which has resulted in the production of disappointing amounts of isobutanol. In this new effort, the researchers looked to optogenetics to provide a more efficient means for getting the yeast to switch between the two phases.
Optogenetics involves programming cells to respond to light. To that end, the researchers added a light-sensitive transcription factor obtained from a marine animal into the yeast. They also introduced changes to another gene, enabling it to block transcription factors. The end result was a yeast that could be switched on and off using light—when light was present, the yeast produced ethanol as normal; when it was turned off, it produced isobutanol.
The researchers found that the modified yeast is capable of producing 8.5g of isobutanol per liter of a glucose solution, which they note is five times more than any other method has delivered. Unfortunately, they note, that amount is still not high enough to make the process commercially useful.
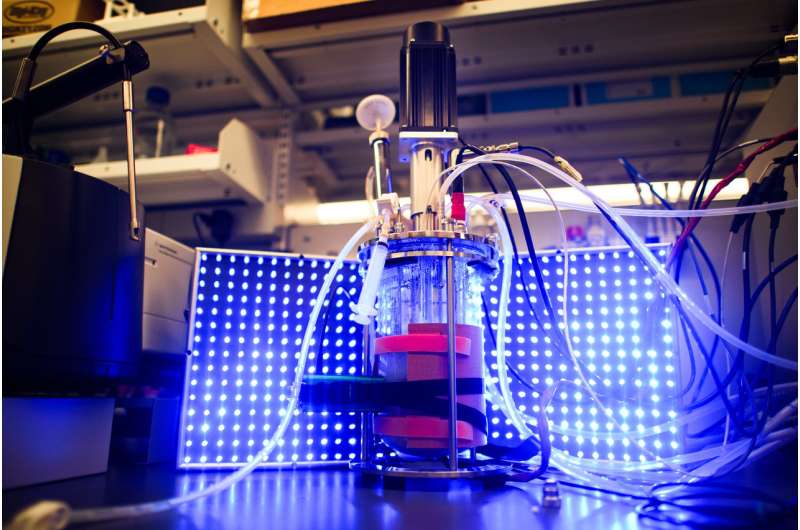
In experiments, researchers used light to control yeast. Credit: Sameer Khan/Fotobuddy
The team plans to continue their research, looking to further optimize the process to see if they can improve yields. They are also considering adding biosensors that can switch the light source on and off automatically, possibly improving efficiency and making the process easier to carry out.
More information: Evan M. Zhao et al. Optogenetic regulation of engineered cellular metabolism for microbial chemical production, Nature (2018). DOI: 10.1038/nature26141
Abstract
The optimization of engineered metabolic pathways requires careful control over the levels and timing of metabolic enzyme expression. Optogenetic tools are ideal for achieving such precise control, as light can be applied and removed instantly without complex media changes. Here we show that light-controlled transcription can be used to enhance the biosynthesis of valuable products in engineered Saccharomyces cerevisiae. We introduce new optogenetic circuits to shift cells from a light-induced growth phase to a darkness-induced production phase, which allows us to control fermentation with only light. Furthermore, optogenetic control of engineered pathways enables a new mode of bioreactor operation using periodic light pulses to tune enzyme expression during the production phase of fermentation to increase yields. Using these advances, we control the mitochondrial isobutanol pathway to produce up to 8.49 ± 0.31 g l−1 of isobutanol and 2.38 ± 0.06 g l−1 of 2-methyl-1-butanol micro-aerobically from glucose. These results make a compelling case for the application of optogenetics to metabolic engineering for the production of valuable products.
Journal information: Nature
© 2018 Phys.org