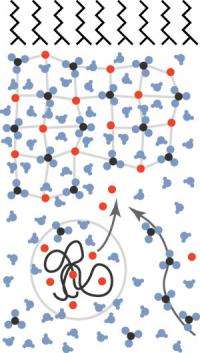
This image depicts the calcium carbonate biomineralization model studied by the research team. The black lines at the top represent the membrane, the network below it is the amorphous, hydrated mineral film and the squiggly black line in the circle is the PAA molecule with its collection of calcium cations (red balls), which get released by the film. The three-atom carbonates (black and blue balls) diffuse from the bottom right. Together, the calcium and carbonate form the mineral film.
Some of the hardest and sturdiest materials aren’t made in the factory; they’re made inside the bodies of animals. Biominerals are commonly used for support and protection, forming in teeth, bones, and shells in animals ranging from humans to mollusks. The cells in an animal’s body have special ways of controlling the sizes and shapes of these mineral compounds and incorporating organic materials into the mix, making many materials that are stronger, harder and more wear-resistant than rocks.
The trick for scientists is to mimic these properties to repair bones and teeth and to find environmentally clean and strong materials for industrial uses. At the NSLS, a group of researchers from Brookhaven and the University of Florida are working toward that goal by studying the process of biomineralization.
“We’re trying to understand how those minerals come to form,” said NSLS physicist Elaine DiMasi. “They’re literally as common seashells on the beach, and the elements they’re made from are very abundant, but synthetically, people have trouble making the particles small enough and getting control over the structure.”
The researchers studied the biomineralization of calcium carbonate by creating a model system with an organic layer to mimic a cell boundary and a liquid designed to precipitate the mineral. Using this model, which was set up in a water trough, the team focused on three factors that control calcium carbonate growth: the presence of magnesium, the presence of polyacrylic acid (PAA), and the escape rate of carbon dioxide.
These three factors were studied at beamline X22B using a special spectrometer that sends x-rays in at an angle and reflects them from different layers in the model system. This allowed the researchers to measure the thickness of the minerals as a function of time while alternating which controls are kept constant.
“We tried to clarify whether these things are all generically the same kind of calcium carbonate inhibitor, or whether they act differently from one another,” DiMasi said. “What we discovered was each has a unique effect that we could differentiate quite clearly.”
For example, the researchers found that PAA, which mimics the presence of proteins, stabilizes the calcium carbonate in the amorphous stage, a disordered phase that is incorporated with water. The amount of time before this calcium carbonate film thins out and dissolves is longest with large amounts of PAA and shortest when there’s a small amount of the polymer in the system.
Another controlling factor, the carbon dioxide escape rate, was studied by altering the depth of the tray containing the model system. Carbon dioxide will diffuse from the system much more quickly with a shallow tray than a deeper one. The team found that this diffusion rate was the variable that controls mineral growth rate.
The final factor, the presence of magnesium, wasn’t found to change the rate of growth as some expected. Instead, its presence causes a delay, or incubation time, in when the calcium carbonate film starts to grow.
Using what they discovered about these three factors, the researchers put together a model of the early formation of calcium carbonate. It goes something like this: PAA has many negative charges associated with it, which pull in positive calcium ions required for the amorphous film to form. Because the calcium is already collected and being held by PAA, the rate of growth depends on the amount of available carbonate, which is produced by the diffusion of carbon dioxide. The more shallow the tray, the quicker carbonate is available and the faster the calcium carbonate amorphous film grows. The magnesium also is attracted to PAA because of its positive charge, and the ion fights against calcium for spots on the polymer. Calcium ultimately binds more strongly to PAA; however, the presence of magnesium in the system delays formation of the film as the two ions compete.
“Previously people thought that magnesium and PAA were both inhibitors, that they both stop calcite from crystallizing from the amporphous precursor phase,” DiMasi said. “Our results show that they do different things and this model shows why. PAA is not just defeating calcite crystal formation; it’s actually stabilizing the amorphous phase by adding cations. And the magnesium is not interfering with crystal formation; it’s interfering with this polymer-prepping action that happens.”
The time-resolved measurements, and small size of the biomineralization system itself, make this a unique study, DiMasi said.
“A lot of people in the field grow crystals and study them in great detail, but we’re looking at the stages before that,” DiMasi said. “Our film might be only 20 nanometers thick while we’re measuring it, and dissolve within 10 hours. If you gave our recipe to someone who needed to grow crystals big enough to look in a microscope, they would probably declare it a failure and rinse it down the sink.”
Source: by Kendra Snyder, National Synchrotron Light Source