This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New model explains precise timing of viral cell bursting
New research from Rice University scientists is shedding light on how viruses ensure their survival by precisely timing the release of new viruses. The discovery offers a new theoretical framework for understanding these dynamic biological phenomena.
The research, published in the Biophysical Journal on July 18, also reveals how certain biological processes achieve remarkable precision despite relying on random events.
"Our findings provide insights into mechanisms crucial for life, from bacteria to humans," said Anatoly Kolomeisky , professor of chemistry, chemical and biomolecular engineering and co-author of the study.
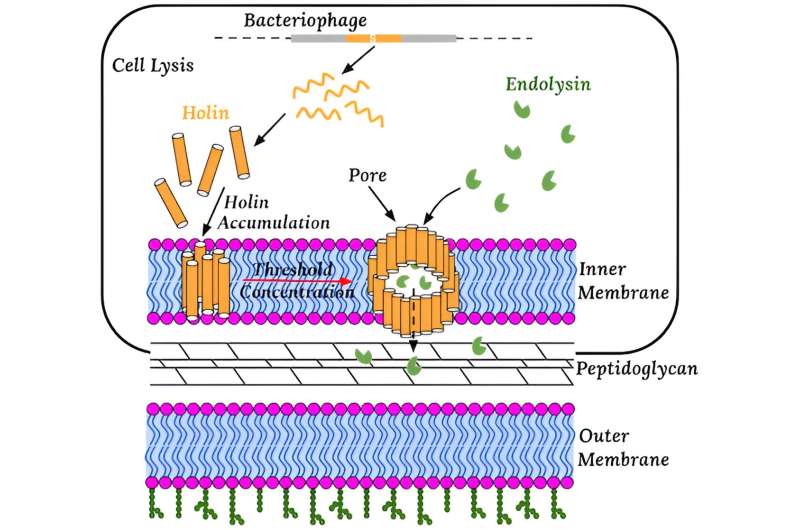
A key focus for the research team was cell lysis, the process by which viruses cause bacterial cells to burst open at just the right moment to release new viruses. This precise timing, essential for viral replication, has puzzled scientists for years.
The research team hypothesized that this precision results from the interplay between two random processes: the buildup of holin proteins, or small phage-encoded membrane proteins that permeabilize the host membrane at a programmed time, and the breaking of the cell membrane.
The researchers proposed that randomly determined coupling between these biophysical and biochemical processes leads to noise cancellation, enabling precise timing.
To test their hypothesis, the researchers developed a mathematical model to analyze the dynamics of holin proteins. They compared their calculations with experimental data from normal and mutated viruses, examining how these proteins accumulate and trigger cell bursting.
Their analysis revealed that precise timing is achieved by maximizing the number of holin proteins in the membrane while maintaining a narrow distribution. This balance ensures that cell lysis occurs at the optimal moment despite the underlying randomness of the processes involved.
"Previous studies had not explored this specific interaction between protein buildup and cell bursting, making the team's findings particularly significant," Kolomeisky said.
The researchers' theoretical predictions aligned closely with experimental observations for wild-type and mutated viruses. They found that wild-type viruses achieve precise timing by balancing the entry and exit of holin proteins in the membrane, while mutated viruses fail to maintain this balance.
The research also highlights how biological systems can achieve precise outcomes through seemingly chaotic processes, and it provides broader insights into how other biological processes might be controlled. By understanding these timing mechanisms, scientists can learn more about fundamental life processes and develop innovative ways to combat bacterial infections.
The research also underscores the intricate balance nature can achieve, ensuring vital processes occur with remarkable accuracy even when influenced by random processes, said Anupam Mondal, co-author of the paper and a postdoctoral fellow at the Center for Theoretical Biological Physics (CTBP).
"The team's work is a step in better understanding the detailed mechanisms of cell lysis, revealing that nature's precision often emerges from the interplay of randomness and regulation," Mondal said.
More information: Anupam Mondal et al, Molecular mechanisms of precise timing in cell lysis, Biophysical Journal (2024). DOI: 10.1016/j.bpj.2024.07.008
Journal information: Biophysical Journal
Provided by Rice University