This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Unlocking the power of nanopores: New design approach scales up opportunities for single-molecule analytics
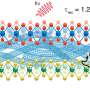
Transmembrane β-barrel pores (TMBs) are extensively used for single-molecule DNA and RNA sequencing. They enable the miniaturization of a wide array of sensing and sequencing applications into portable USB-size devices and point-of-care technologies. A team of Belgian and American researchers has now described a general approach to designing TMB pores from scratch with custom shapes and properties, opening up new opportunities for single-molecule analytics. Their results were published in Science.
Protein nanopores are the holy grail in the field of analytical biology. These nanometer-sized proteins form regular pores in lipid membranes and are widely used for single-molecule DNA and RNA sequencing. They hold a considerable potential to advance a broad range of sensing and sequencing applications by taking them out of specialized labs and into portable devices. However, current approaches to engineering nanopore sensors are limited to naturally occurring proteins, which have evolved for very different functions and are less than ideal starting points for sensor development.
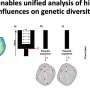
Research led by the VIB-VUB Center for Structural Biology (Belgium) and the University of Washington School of Medicine (U.S.) has taken on the challenge of designing these protein "barrels" from scratch, with the ultimate goal of controlling the shape and chemistry on a molecular level.
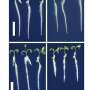
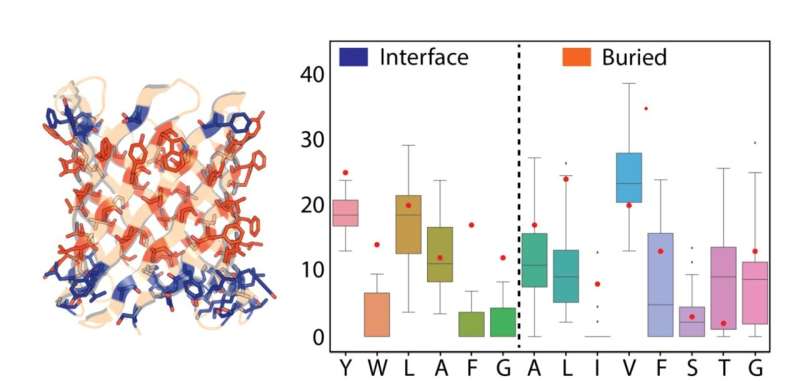
With the help of computational design, the researchers developed methods to design stable nanopore channels with tunable pore shapes, sizes, and conductance. Compared to natural pores, the signal generated by the designed TMBs was remarkably stable and quiet. Collaborators in the laboratory of Sheena Radford (University of Leeds) and Sebastian Hiller (Biozentrum, University of Basel) found that the designs folded into stable 3D structures. This opens the door to designing nanopore channels de novo that are suitable for many applications of interest in research and industry.
"These developments are very exciting. When we started with this idea a few years ago, many people thought it was impossible, because the design and folding of β-sheets is incredibly complex, let alone in lipid membranes. Now we have shown that we can successfully design nanopores with a high success rate, which have stable and reproducible conductance," says Dr. Anastassia Vorobieva, group leader at the VIB-VUB Center for Structural Biology.
As the next step, the researchers put their design method to the test. Nanopores that can detect very small molecules such as metabolites would be extremely useful tools for metabolomic and diagnostic analysis, which currently requires large, specialized lab equipment. The design of functional small-molecule sensors remains challenging because of the complexity of protein-ligand interactions. Hence, the pores must have a highly complementary shape to the small molecule of interest.
A team from the laboratory of UW Medicine biochemistry professor and HHMI Investigator David Baker successfully designed new proteins that can specifically bind small-molecule metabolites. They split the proteins into three parts and fused the parts into the loops of a TMB pore. They found that they could directly detect single-molecule binding events using such constructs.
"This collaboration is a great example of what's possible with protein design. Rather than repurposing biomolecules from nature, we can now create the functions we want from first principles," remarks Prof. Dr. David Baker, professor at the University of Washington School of Medicine and HHMI investigator.
The positive results prove that nanopore design can complement mass spectrometry and other analytical methods that require big labs and big setups because the technology is smaller and more accessible. Although we are still quite a bit removed from this point, the researchers envision a future in which portable devices with different nanopores can sense a range of metabolites, proteins, and small molecules, or even do biomolecular sequencing.
More information: Samuel Berhanu et al, Sculpting conducting nanopore size and shape through de novo protein design, Science (2024). DOI: 10.1126/science.adn3796. www.science.org/doi/10.1126/science.adn3796
Journal information: Science
Provided by VIB (the Flanders Institute for Biotechnology)