This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Material with molecular trapdoor holds promise for highly selective gas adsorption
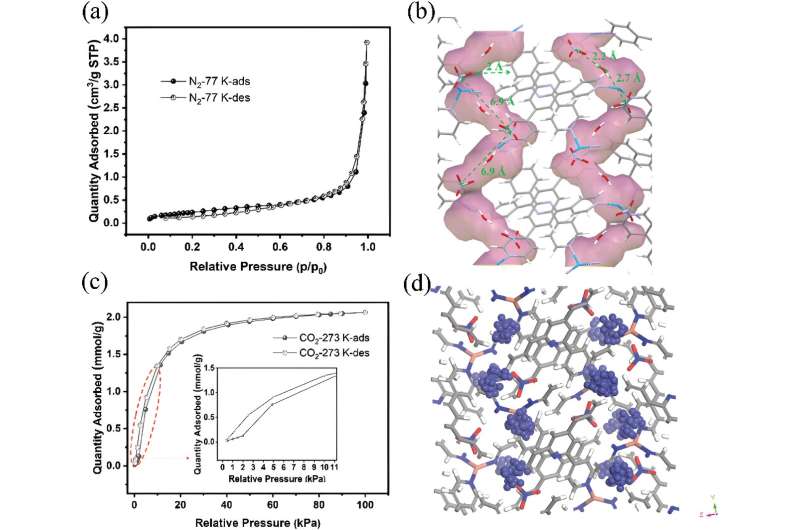
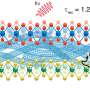
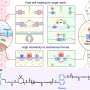
An international team led by scientists at City University of Hong Kong has found a flexible metal-organic framework (MOF) with one-dimensional channels that acts as a "molecular trapdoor" to selectively adsorb gases, such as carbon dioxide, in response to temperature and pressure changes.
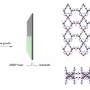
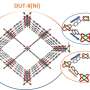
In work published in Small and featured on the back cover of the journal, investigators including principal beamline scientist Dr. Qinfen Gu at the Australian Synchrotron, reported a solid one-dimensional channel ionic metal organic framework (MOF) had internal voids that could hold gas molecules.
They discovered a temperature-sensitive gas adsorption behavior similar to the "molecular trapdoor" effect as discovered by the lead authors a decade ago in CHA zeolite, a mineral with a three-dimensional framework structure.
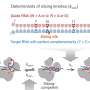
Extra-framework anions, negatively charged ions that are not part of the main structural framework of a material, act as "gates" that shift from their usual positions under heat or pressure, allowing gas to enter.
Gas separation is crucial in numerous industrial contexts, including applications like natural gas separation, carbon capture, and hydrogen purification, among others. However, achieving high gas selectivity is difficult when gases are similar in size and share properties.
Different guest molecules create varying energy barriers for the deviation of anions (momentary gate-opening) in pyridine-based ligands, enabling gas separation based on different specific threshold temperatures for exclusive gas admission.
Larger, spherical anions improved the selectivity for gases like greenhouse gas CO2/CH4, hydrocarbons C3H6/C3H8 and natural gas N2/CH4.
Although this was observed at low temperatures and with limited separation capabilities, the investigators suggested that the concept can be applied to design other types of MOFs. The finding may lead to the creation of "molecular trapdoor" MOFs with high surface areas that offer better capacity and separation performance.
"This finding challenges the traditional view that gas adsorption is only about gas affinity or size-based sieving, highlighting the role of guest-induced anion movements in gas diffusion within MOFs," said Prof Jin Shang, Associate Professor in the School of Energy and Environment, City University of Hong Kong, who supervised first author Yuanmeng Tian.
The flexibility of the MOFs with respect to temperature was carefully investigated, as temperature-triggered gate-opening behavior in MOFs is typically associated with structural transformation.
In situ single crystal X-ray diffraction experiments and in situ synchrotron powder X-ray diffraction measurements were performed at different temperatures to identify the topological flexibility of MOFs using the macromolecular crystallography and powder diffraction beamlines at the Australian Synchrotron.
"XRD diffraction was used to gain an understanding of the flexibility of the structural framework and movement of anions as gate-keepers in response to temperature, as well as confirm the success of anion exchange and determine the exchange rate," explained Dr. Gu.
Macromolecular crystallography beamline scientist Dr. Stefanie Bird assisted with the single crystal measurements that provided information about structural changes at different temperatures.
More information: Yuanmeng Tian et al, Tunable Gas Admission via a "Molecular Trapdoor" Mechanism in a Flexible Cationic Metal–Organic Framework Featuring 1D Channels, Small (2024). DOI: 10.1002/smll.202400064
Journal information: Small