This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Atomically controlled MXenes enable cost-effective green hydrogen production
A total of 137 countries around the world have signed a "net-zero" climate change agreement to end fossil fuel use and achieve zero carbon emissions by 2050. Hydrogen is being touted as the next green energy source because it emits only water and oxygen when utilized as an energy source.
Hydrogen production methods are divided into gray hydrogen, blue hydrogen, and green hydrogen depending on the energy source and carbon emissions. Among them, green hydrogen production method is the most eco-friendly method that produces hydrogen without carbon emissions by electrolyzing water using green energy.
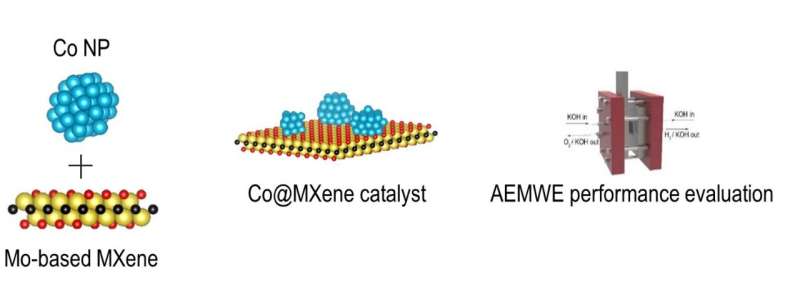
A research team led by Dr. Albert Sung Soo Lee of the Convergence Research Center for Solutions to Electromagnetic Interference in Future-Mobility and Materials Architecturing Research Center at Korea Institute of Science and Technology (KIST), in collaboration with Professor Chong Min Koo's group at Sungkyunkwan University, has developed an oxidatively stable molybdenum-based MXene as electrocatalyst support in anion exchange membrane water electrolyzers.
The study is published in the journal Applied Catalysis B: Environment and Energy.
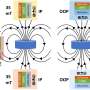
As it is stable against oxidative high voltage conditions, if it is applied as a carrier for electrolysis catalysts, it can be used as an oxygen evolution reaction electrode material for green hydrogen production to reduce the cost of green hydrogen production.
The breakdown of water into hydrogen and oxygen molecules requires a high amount of energy. To reduce this initial reaction energy, a catalyst is used, and the smaller size of the catalyst, which is made up of tiny nanoscale particles, the larger the surface area, which allows the reaction to take place.
However, over time, small catalyst particles can agglomerate, reducing the surface area and reducing the efficiency of hydrogen production. To prevent this, catalysts and supports are used together, and carbon is mainly used for the cathode, where hydrogen is produced, but when carbon is used in an oxidation reaction at the anode, it is oxidized to carbon dioxide. Thus, a support with high oxidation resistance is required.
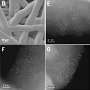
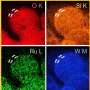
One material that can be used as a support is MXene. MXenes are nanomaterials composed of metal atoms (Ti, Mo, Hf, Ta, etc.) and carbon or nitrogen atoms, which show electrically conductive properties and have a 2D nanostructure suitable for catalyst support, making them favorable for hydrogen production.
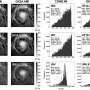
Titanium-based MXenes have been the most widely studied due to their high electrical conductivity. However, the atomic nature of titanium, which is easily oxidized in water, has led to the inherent disadvantage that the catalyst cannot maintain high electrical conductivity. To overcome this, the team designed a new anode catalyst that uses molybdenum-carbide based MXene as a support.
-

An electrode with a molybdenum MXene catalyst transferred onto an electrolyzer device. The cathode element, one of the key components of a hydrogen production device, is being held. Credit: Korea Institute of Science and Technology -

(Standing from left) Senior Research Scientist Dr. Albert Sung Soo Lee, Postdoctoral Researcher Gwan-Hyun Choi, and (Sitting) Student Researcher Young Sang Park at KIST. Credit: Korea Institute of Science and Technology
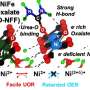
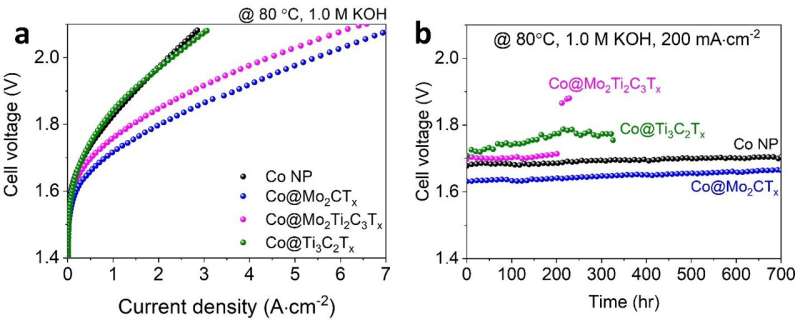
When the molybdenum-based MXene is utilized as a support, strong chemical bonds are created between the molybdenum atoms on the surface of the MXene and the active materials cobalt.
The resulting chemical bonds increased the hydrogen production efficiency by about 2.45 times. In particular, the durability of the unit cell was improved by more than 10 times compared to the results of a recent titanium-based MXene, which lasted less than 40 hours.
This is expected to reduce the cost of green hydrogen production and will be applied to large-scale hydrogen production plants and large-scale green hydrogen power stations in the future.
"By controlling the elements that make up MXene, we were able to find suitable candidates for green hydrogen production environments, and through this, we secured a stable MXene support in an oxidizing environment," said Dr. Albert Sung Soo Lee of KIST.
"In the future, we will contribute to the revitalization of hydrogen-based economy by developing oxygen-generating electrode catalysts with catalytic efficiency and durability."
More information: Young Sang Park et al, Unveiling the role of catalytically active MXene supports in enhancing the performance and durability of cobalt oxygen evolution reaction catalysts for anion exchange membrane water electrolyzers, Applied Catalysis B: Environment and Energy (2024). DOI: 10.1016/j.apcatb.2024.123731