This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Magnetism boosts hydrogen production in model catalysts
Researchers at the University of Twente have shown how to improve the efficiency of hydrogen production in an experimental setup. They showed that the magnetic order of the molecules plays a critical role.
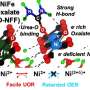
In the search for green hydrogen, the design of efficient catalyst materials that increase the efficiency and speed of the chemical reaction that produces (green) hydrogen is essential. Theoretically, several studies already implied that the magnetic properties of the catalyst affect this efficiency and speed. However, experimental evidence for these enhancements has been sparse, especially for the role of magnetic properties which exist without external magnetic fields—until now.
The researchers aligned the magnetic "spins" of the atoms in the catalyst while the reaction was happening. They saw that aligning all these tiny magnets increased the reaction speed. "Although we're working on a fundamental scale, this research can have important implications for efficient hydrogen production," says first author Emma van der Minne. The findings are published in the journal Applied Physics Reviews.
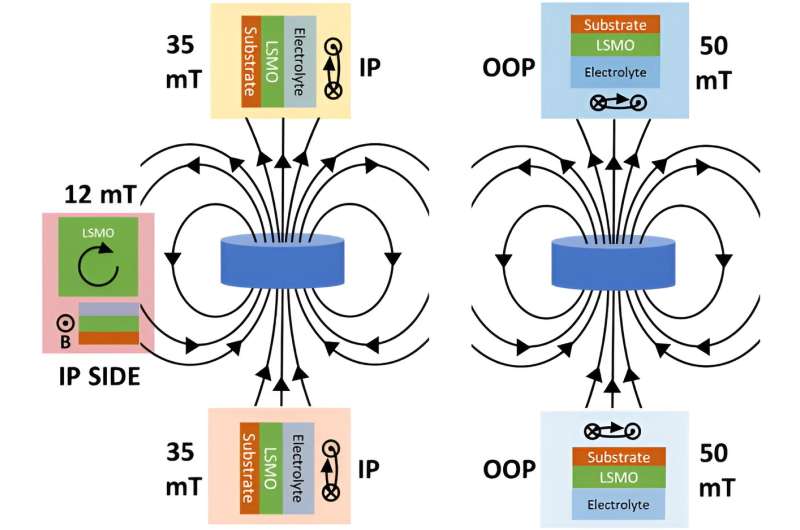
To align the magnetic spins while the reaction was running, the researchers used a simple approach. Van der Minne explains, "We decreased the temperature during the reaction. By comparing the changes in the reaction speed during this decrease for two catalysts with a different magnetic state, we found that the activity is really increased by this so-called magnetic order. The fact that this happens even without the application of a magnetic field was unclear before. Our research thus gives us new ways to improve the design of catalysts in large-scale hydrogen production."
But that's not all. The researchers also found that if they applied an external magnetic field, it made the catalyst even better at its job. The direction of this magnetic field mattered. It had to line up just right with the material's magnetic properties. Understanding how both the magnetism inside the catalyst and its reaction to external magnetic fields affect the reactions involved in hydrogen production brings us one step closer to a greener future.
More information: Emma van der Minne et al, The effect of intrinsic magnetic order on electrochemical water splitting, Applied Physics Reviews (2024). DOI: 10.1063/5.0174662
Journal information: Applied Physics Reviews
Provided by University of Twente