This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Research team develops catalyst that can purify municipal sewage while enhancing hydrogen generation efficiency
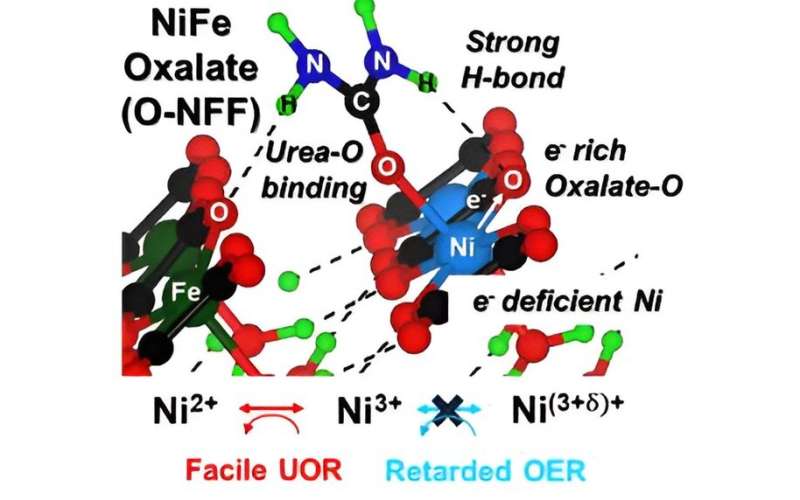
Researchers have devised a novel catalyst aimed at enhancing the efficiency of reactions using contaminated municipal sewage to produce hydrogen—a green energy source.
Their research was recently featured in Advanced Functional Materials.
With the growing environmental concerns of pollution associated with fossil fuel, hydrogen has garnered increased interest. Water electrolysis technology is a sustainable process that leverages Earth's abundant water to produce hydrogen. However, the concurrent oxygen evolution reaction during hydrogen production is notably slow, resulting in a considerably low energy conversion efficiency.
Lately, the academic community has been tackling this issue by integrating the urea oxidation reaction with the hydrogen generation reaction. Urea, a pollutant found in urine, releases a significant amount of energy during its oxidation process, offering a potential means to enhance both the efficiency of hydrogen generation and the purification of toilet wastewater.
Ultimately, it is necessary to find a catalyst that can effectively drive the urea oxidation reaction, thereby amplifying the efficiency of both hydrogen generation and wastewater treatment.
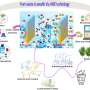
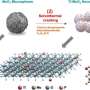
In pursuit of increased efficiency in the urea oxidation reaction, the team created a catalyst known as nickel-iron-oxalate (O-NFF). This catalyst combines iron (Fe) and oxalate on nickel (Ni) metal, resulting in an expansive surface area characterized by nanometer-sized particles in fragment form. This unique property enables the catalyst to adsorb more reactants, facilitating an accelerated urea oxidation reaction.
In experiments, the O-NFF catalyst devised by the team successfully lowered the voltage required for hydrogen generation to 1.47 V RHE (at 0.5 A/cm2) and exhibited a high reaction rate even when tested in a mixed solution of potassium hydroxide (1 M) and urea (0.33 M) with a Tafel slope of 12.1 mV/dec.
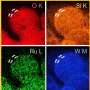
The researchers further validated the catalyst's efficacy by confirming its promotion of the urea oxidation reaction through photoelectron/X-ray absorption spectroscopy using a radiation photon accelerator.
Professor Kangwoo Cho and Ph.D. candidate Jiseon Kim from the Division of Environmental Science & Engineering at Pohang University of Science and Technology (POSTECH) collaborated with the Korea Institute of Science and Technology (KIST) for this study.
Cho, who led the research, stated, "We have developed a catalyst capable of purifying municipal sewage while simultaneously enhancing the efficiency of hydrogen production, a green energy source. We anticipate that O-NFF catalysts, synthesized from metals and organics, will contribute to the improved efficiency of industrial electrolysis hydrogen production."
More information: Jiseon Kim et al, Accessible Ni‐Fe‐Oxalate Framework for Electrochemical Urea Oxidation with Radically Enhanced Kinetics, Advanced Functional Materials (2024). DOI: 10.1002/adfm.202315625
Provided by Pohang University of Science and Technology