This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New material opens up possibility of converting water pollutants into hydrogen gas
WPI Researchers have developed a material to remove urea from water and potentially convert it into hydrogen gas. By building these materials of nickel and cobalt atoms with carefully tailored electronic structures, the group has unlocked the potential to enable these transition metal oxides and hydroxides to selectively oxidize urea in an electrochemical reaction.
The study, led by Xiaowei Teng, the James H. Manning professor of Chemical Engineering at WPI, was published in the Journal of Physical Chemistry Letters and highlighted in the publication's supplementary front cover.
The challenge of removing urea from water
Urea is a low-cost nitrogen agriculture fertilizer and a natural product from human metabolism. Urea-rich agricultural runoff and municipal wastewater discharge cause eutrophication–harmful algal blooms and hypoxic dead zones that adversely impact the aquatic environment and human health.
At the same time, the unique characteristics of urea make it a potential hydrogen storage medium that could offer viable on-demand hydrogen production. For instance, urea is non-toxic, has high water solubility, and has high hydrogen content (6.7% by weight). Thus, urea electrolysis for hydrogen production is more energy-efficient and economical than water electrolysis.
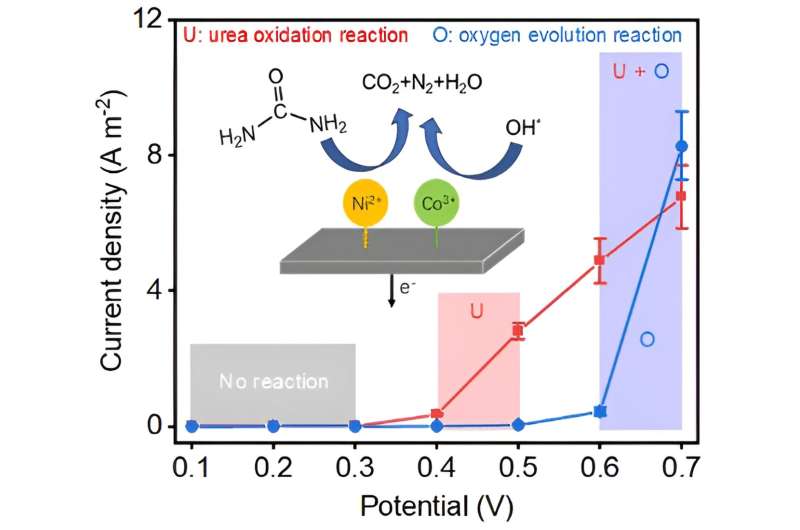
The weakness of urea electrolysis has always been the lack of low-cost and highly efficient electrocatalysts that selectively oxidize urea instead of water, but Teng and his team have found a solution: making electrocatalysts consisting of synergistically interacted nickel and cobalt atoms with unique electronic structures for selective urea electro-oxidation.
Unlocking enhanced selectivity and activity
The WPI team's study centered on homogeneous nickel and cobalt oxides and hydroxides. Researchers found that the key to enhancing its electrochemical activity and selectivity to urea oxidation lay in tailoring the unique electronic structures with dominant Ni2+ and Co3+ species.
"This electronic configuration is a pivotal factor to improve the selectivity of urea oxidation because we observe that higher nickel valence, such as Ni3+, indeed helps produce a fast reaction with strong electric current output; however, a large portion of current was from unwanted water oxidation," Teng said.
To better understand this effect, Teng's group collaborated with Aaron Deskins, a professor of chemical engineering at WPI. Deskins performed the computational simulations and found that homogenous mixing of nickel and cobalt oxides and hydroxides benefited the electron redistribution from Ni2+ to Co3+ species and shifting valence electrons to higher energy so the Ni/Co catalysts were better prepared to participate in bonding with urea and water molecules.
Applications and future prospects
A major nitrogen fertilizer and feed additive, urea was commercially produced as early as the 1920s; around 180 million metric tons were produced in 2021. Urea can be derived from natural sources; an adult human produces 1.5 L of urine daily, equivalent to 11 kg of urea and 0.77 kg of hydrogen gas yearly.
The team's findings could help use urea in waste streams to efficiently produce hydrogen fuel through the electrolysis process, and could be used to sequester urea from water, maintaining the long-term sustainability of ecological systems, and revolutionizing the water-energy nexus.
More information: Tongxin Zhou et al, Enhanced Urea Oxidation Electrocatalytic Activity by Synergistic Cobalt and Nickel Mixed Oxides, The Journal of Physical Chemistry Letters (2023). DOI: 10.1021/acs.jpclett.3c03257
Journal information: Journal of Physical Chemistry Letters
Provided by Worcester Polytechnic Institute