This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Efficient bi-functional catalyst for methanol-assisted water splitting of hydrogen generation
Hydrogen energy, as a green and environmentally friendly energy carrier, is a critical switch in energy conversion to the green economy, and the green hydrogen generation via water splitting technique from renewable energy sources is regarded as the best choice. While, the high energy input for water splitting is thermodynamically required (1.23 V), and the cell voltage can go beyond 1.8 V for the practical water electrolysis resulting from the retard kinetics of oxygen evolution reaction (OER).
The use of low oxidation potential methanol (0.016 V) assisted water electrolysis has received much attention as it can match renewable energy sources to achieve hydrogen production with low energy consumption.
The key to realizing this sustainable vision of green hydrogen production is proposed to explore the efficient bifunctional catalysts for catalytic methanol oxidation and hydrogen evolution reaction. However, the traditional Pt-based catalyst surface is susceptible to poisoning by the adsorbed CO intermediates (CO*), resulting in sluggish reaction kinetics and catalytic performance degradation, which severely restricts the development of methanol electrolysis for hydrogen production.
The introduction of oxophilic function components can promote the dissociation of water at low potentials and generate more oxygen-containing species, which can assist the removal of CO* on the Pt surface, and thus significant improvement in the activity and stability for MOR.
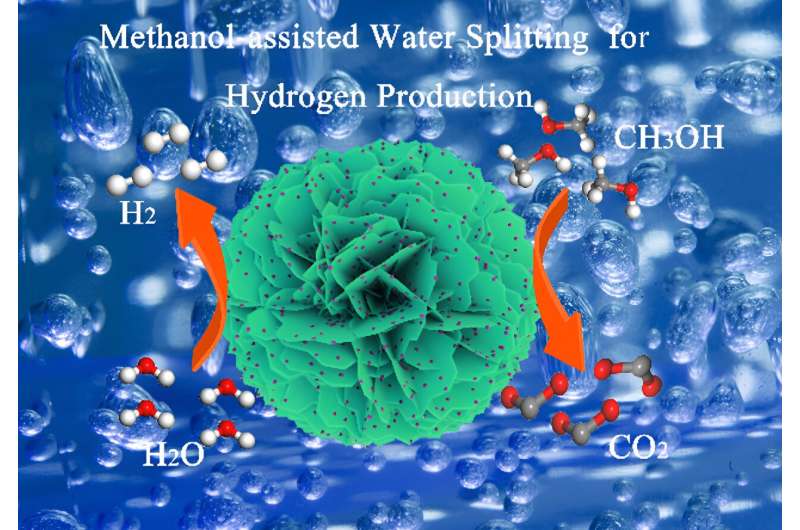
Recently, a research team led by Prof. Ligang Feng from Yangzhou University, China, reported a new platform, MoSe2 nanosheets supported Pt nanoparticles, for the methanol-assisted water splitting catalyzed by a bi-functional Pt/MoSe2 system for hydrogen generation.
The oxophilic MoSe2 component with 2D structures optimized the adsorption of CO* and H* on Pt sites as demonstrated by spectroscopic and theoretical analysis, which resulted in enhanced catalytic ability in methanol-assisted water splitting reaction. The peak current density was 2.5 times higher than that of commercial Pt/C catalyst for methanol oxidation and a small overpotential of 32 mV was demanded to achieve a current density of 10 mA cm–2 for hydrogen evolution reaction in the methanol-containing electrolyte.
When serviced as both cathode and anode, a low cell voltage of 0.66 V was required at 10 mA cm–2, significantly lower than that of 1.75 V required for water splitting. The results were published in Chinese Journal of Catalysis.
More information: Wei Qiao et al, Efficient bi-functional catalysis of coupled MoSe2 nanosheet/Pt nanoparticles for methanol-assisted water splitting, Chinese Journal of Catalysis (2023). DOI: 10.1016/S1872-2067(23)64469-9
Provided by Chinese Academy of Sciences