Discovery of a new catalyst for highly active and selective carbon dioxide hydrogenation to methanol
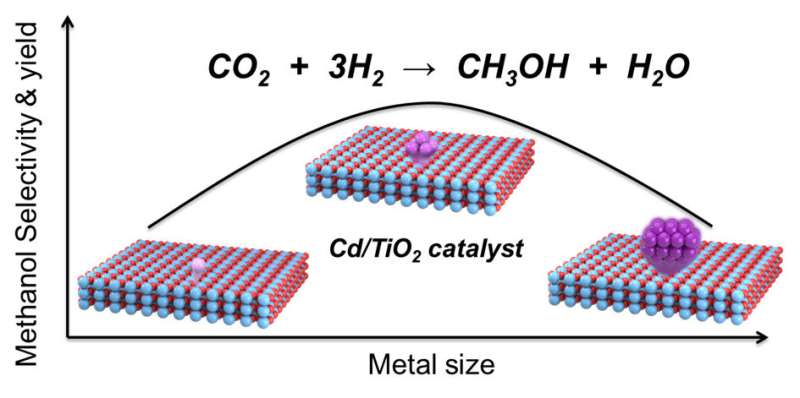
A new catalyst of Cd/TiO2, enabling 81% methanol selectivity at 15.8% CO2 conversion with the CH4 selectivity below 0.7% was discovered. The combination of experimental and computational studies show that the unique electronic properties of Cd cluster supported on TiO2 are responsible for the high selectivity for CO2 hydrogenation to methanol via a HCOO* pathway realized at the interface catalytic sites.
Carbon dioxide capture and utilization (CCU) using renewable energy is an effective way to achieve carbon neutrality, thus drawing increasing attention from industry and academia worldwide. A promising route for CO2 utilization is methanol production (CO2 + 3H2 → CH3OH + H2O) since methanol can be used as an easily transportable fuel, an H2-storage molecule, or a precursor for the production of olefins and aromatics. Heterogeneous catalysts are commonly available for CO2 hydrogenation to methanol by using a fixed bed reactor, which is capable for scale-up industrial applications. Until now, CuZnO catalysts have been widely investigated for CO2 hydrogenation to methanol. However, the methanol selectivity reported so far hardly exceeds 60% under the optimal operation conditions because of the competing side reactions, such as reverse water-gas shift reaction (RWGS). Besides, Cu-based catalysts usually suffer from deactivation caused by sintering. Thus, non-Cu catalysts have drawn increasing attentions in recent years.
Recently, a research team led by Prof. Can Li from Dalian Institute of Chemical Physics, Chinese Academy of Sciences, China discovered a Cd cluster based Cd/TiO2 catalyst, which shows 81% methanol selectivity at CO2 conversion of 15.8%, while enabling to keep the CH4 under 0.7% at 5 MPa. The results were published in Chinese Journal of Catalysis.
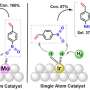
Cd/TiO2 catalysts were prepared by wet impregnation using TiO2 support. Both the activity and selectivity increase with the increase of Cd loading and reach the maximum at 3.5%Cd. Further increasing the Cd loading from 3.5% to 7% just results in a slight decrease of both activity and selectivity. 3.5%Cd/TiO2 catalyst exhibits 81% methanol selectivity and 15.8% CO2 conversion under 5 MPa, and exhibits a methanol yield of 6.7% (X(CO2)=9.4%, S(CH3OH)=71%), which is approaching the thermodynamic equilibrium under the conditions of 2 MPa, 290 oC.
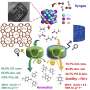
The structure characterizations show that Cd species of 0.35%Cd/TiO2 catalyst are atomically dispersed with isolated Cd sites on TiO2. For 3.5%Cd/TiO2 catalyst, a lot of sub-nanometer Cd clusters emerge besides isolated Cd sites. When the Cd loading further increases to 7%, nanometer-size Cd particles are observed in addition to Cd clusters and isolated Cd sites. During the reaction, the Cd species are in the +2 oxidation state for 0.35%Cd/TiO2 and 3.5% Cd/TiO2, Cd species are reduced to metal state for 7%Cd/TiO2.
The mechanism investigations show that the HCOO* pathway is a possible pathway for CO2 hydrogenation to methanol. DFT calculations show that the key reaction intermediates of HCOO*, HCOOH*, and CH2O*_H2O* on the surface of CdTiO3 (Cd1 structure) are much more stable compared to the respective states on the Cd4/TiO2 interface. Accordingly, the evolution of these intermediates along with the catalytic reaction coordinate proceeds with much higher barriers, evidencing a much higher catalytic CO2 hydrogenation activity of the Cd4/TiO2 over the bulk CdTiO3 mixed oxide phase.
More information: Jijie Wang et al, Highly dispersed Cd cluster supported on TiO2 as an efficient catalyst for CO2 hydrogenation to methanol, Chinese Journal of Catalysis (2022). DOI: 10.1016/S1872-2067(21)63907-4
Provided by Chinese Academy of Sciences