This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers develop iron complex catalyst for selective and efficient conversion of methane to methanol
Extensive research has been conducted on the oxidation of methane to obtain methanol for the production of useful compounds, such as formaldehyde, dimethyl ether, etc. However, methane is the most difficult hydrocarbon to be oxidized, and to date, to the best of our knowledge, no method has been developed to efficiently and selectively convert methane to methanol under mild conditions.
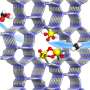
Inspired by the structure and reaction mechanism of naturally occurring metalloenzymes that oxidize methane, researchers have developed a mononuclear iron complex with a hydrophobic environment near the active iron center. Using this iron complex as a catalyst and a cheap and safe oxidant, they have accomplished the direct conversion of methane to methanol with high efficiency and selectivity.
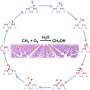
In this reaction, the oxidation of methane proceeds under mild conditions of 50 °C temperature and ~10 atm pressure in an aqueous solution. The catalytic turnover number exceeds 500 in 3 h and methanol can be produced with a high selectivity of 83%.
The achievement of such high efficiency and selectivity is owing to the presence of a hydrophobic environment near the iron center, i.e., the active site of the catalyst. The hydrophobic cavity traps methane (hydrophobic substance) and releases the generated methanol (hydrophilic substance) into the aqueous solution while preventing the excessive oxidation of methanol by suppressing its approach to the active iron center.
Such a "catch-and-release" mechanism is expected to be an effective model that can be applied to the conversion of methane to methanol as well as to the efficient chemical conversion of various hydrophobic organic compounds.
The study is published in the journal Nature.
More information: Hiroto Fujisaki et al, Selective methane oxidation by molecular iron catalysts in aqueous medium, Nature (2023). DOI: 10.1038/s41586-023-05821-2
Journal information: Nature
Provided by University of Tsukuba