Researchers convert methane to formic acid at high efficiency under mild conditions
Methane is promising energy resources for producing high-value-added chemicals. Methane conversion to value-added chemicals or fuels under mild conditions has become one of the hottest topics in energy and catalysis.
However, the high symmetry and low polarizability of methane molecule make it challenging to activate methane under mild conditions. In addition, the target products are usually more reactive than the methane, and they are subject to over-oxidation to the greenhouse gas CO2.
Recently, a group led by Prof. DENG Dehui and Assoc. Prof. Yu Liang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) converted methane to formic acid (HCOOH) at high efficiency under mild conditions. Their study was published in Nano Energy on Dec. 29.
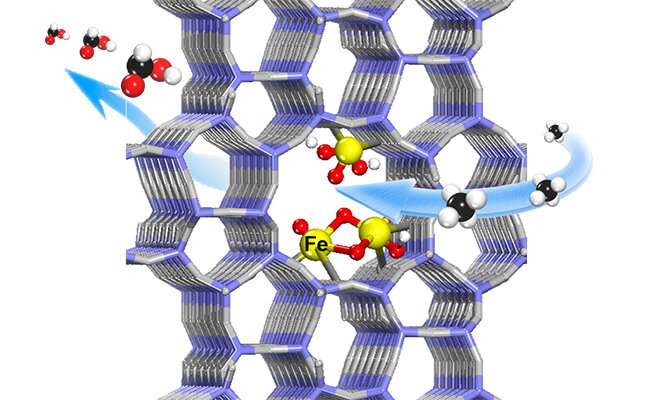
The researchers found that highly efficient and selective oxidation of methane to HCOOH could be achieved on the atomically-dispersed Fe sites confined within the channels of ZSM-5.
By adjusting the silica-alumina ratio and Fe loading in the ZSM-5 to modulate the microenvironment of the active Fe species, they reached a turnover frequency (TOF) of 84200 h-1 for producing C1 oxygenates and a high HCOOH selectivity of 91% with productivity of 383.2 mmol gcat.-1 h-1 at 80 degrees C.
"This result surpassed all previously reported methane conversion catalysts operated under similar conditions," said Prof. DENG.
Furthermore, by combining various characterizations and density functional theory calculations, they found that Fe-O active sites could be generated from H2O2 dissociation on both the mononuclear and binuclear Fe centers confined within the channels of ZSM-5.
The Fe-O active sites could facilitate the activation and cleavage of the C-H bond of methane and thereby promoted successive oxidation of methane to formic acid through methanol and formaldehyde via free radical pathways, while inhibiting the over-oxidation to CO2.
This study paves a new route to design and construct lattice-confined active sites for highly efficient conversion of methane under mild conditions.
More information: Kaixin Zhu et al. Highly efficient conversion of methane to formic acid under mild conditions at ZSM-5-confined Fe-sites, Nano Energy (2020). DOI: 10.1016/j.nanoen.2020.105718
Journal information: Nano Energy
Provided by Chinese Academy of Sciences