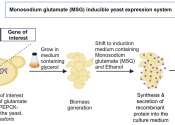
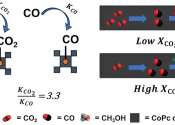
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH (often abbreviated MeOH). It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol (drinking alcohol). At room temperature, it is a polar liquid, and is used as an antifreeze, solvent, fuel, and as a denaturant for ethanol. It is also used for producing biodiesel via transesterification reaction.
Methanol is produced naturally in the anaerobic metabolism of many varieties of bacteria, and is ubiquitous in the environment. As a result, there is a small fraction of methanol vapor in the atmosphere. Over the course of several days, atmospheric methanol is oxidized with the help of sunlight to carbon dioxide and water.
Methanol burns in air, forming carbon dioxide and water:
Because of its toxic properties, methanol is frequently used as a denaturant additive for ethanol manufactured for industrial uses — this addition of methanol exempts industrial ethanol from liquor excise taxation. Methanol is often called wood alcohol because it was once produced chiefly as a byproduct of the destructive distillation of wood.