This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Novel catalysts for improved methanol production using carbon dioxide dehydrogenation
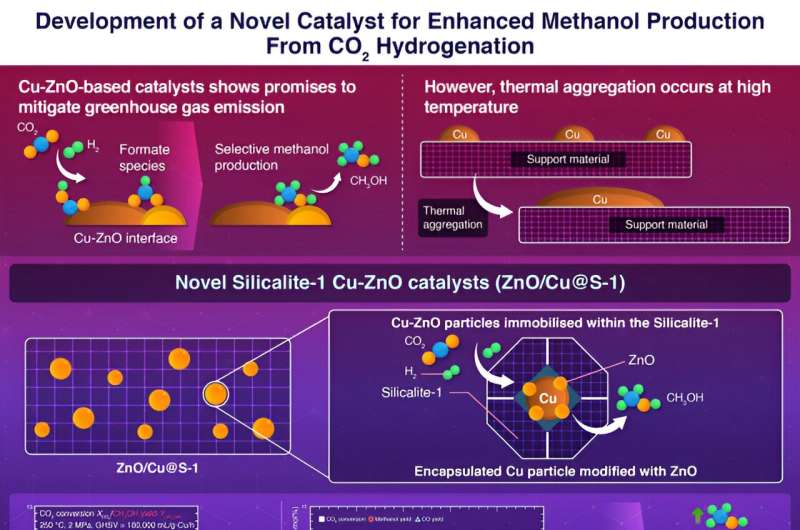
Encapsulating copper nanoparticles within hydrophobic porous silicate crystals has been shown by scientists at Tokyo Tech to significantly enhance the catalytic activity of copper-zinc oxide catalysts used in methanol synthesis via CO2 hydrogenation.
The innovative encapsulation structure effectively inhibits the thermal aggregation of copper particles, leading to enhanced hydrogenation activity and increased methanol production. This breakthrough paves the way for more efficient methanol synthesis from CO2.
Carbon dioxide (CO2) emissions are a major contributor to global warming, highlighting the pressing need for emission reduction measures. Consequently, researchers are exploring alternatives to fossil fuels, the primary source of CO2 emissions.
Methanol emerges as a versatile and cost-effective fuel, offering a promising alternative to conventional transportation fuels. Furthermore, in efforts to mitigate the impact of these emissions, there has been significant attention directed toward CO2 capture and utilization technologies.
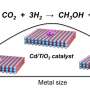
These innovative approaches involve capturing CO2 from the atmosphere and converting it into value-added products. Methanol synthesis via CO2 hydrogenation stands out as a particularly promising method among these technologies.
For methanol synthesis via CO2 hydrogenation, lower reaction temperatures are preferable since heat is released during the reaction. Accordingly, studies have focused on the development of catalysts that exhibit high activities at low temperatures.
Copper-zinc oxide (Cu-ZnO) based catalysts are particularly favorable for this purpose due to their ability to form a Cu-ZnO interface that binds and converts CO2 into formate intermediates which, in turn, promote methanol production. Increasing the surface area of this interface is an effective way to improve production, which can be achieved by increasing the dispersion of Cu nanoparticles.
However, Cu nanoparticles are thermally unstable, which aggregate during catalyst preparation and reaction, thus reducing the interface area. Furthermore, the formation of water as a by-product of methanol synthesis accelerates Cu aggregation and inhibits formate formation.
To address these issues, a team of researchers, led by Professor Teruoki Tago from the Department of Chemical Science and Engineering, School of Materials and Chemical Technology at Tokyo Institute of Technology, developed novel Silicalite-1 (S-1) encapsulated Cu-ZnO catalysts.
"Research indicates that encapsulating metals within porous carriers like silica or zeolite effectively mitigates thermal aggregation. Therefore, our focus shifted to developing a novel and efficient Cu-based catalyst for methanol production via CO2 hydrogenation, placing particular emphasis on encapsulating Cu nanoparticles within porous materials," explained Tago.
Their study was published in the Chemical Engineering Journal, on April 1, 2024.
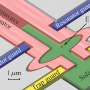
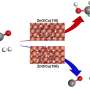
The researchers fabricated two types of catalysts, one including a Cu/S-1 catalyst in which copper was loaded onto hydrophobic S-1 by impregnation, and the other Cu@S-1 catalyst, in which a Cu phyllosilicate (CuPS) powder was used as a metal source to encapsulate Cu particles in the S-1 zeolite.
Cu@S-1 was prepared by reducing dissolved CuPS powder. The researchers investigated the dissolution time of the CuPS precursors on the catalyst properties, revealing that inappropriate dissolution times significantly affect the size of Cu particles.
Optimal dissolution of the precursor resulted in a catalyst with approximately 2.4-nanometer Cu particles encapsulated within S-1, exhibiting optimal catalytic activity. This catalyst demonstrated higher hydrogenation activity and methanol production than Cu/S-1.
To further improve methanol production, ZnO was added to Cu@S-1 by impregnation, forming ZnO/Cu@S-1 catalyst with fine Cu particles. This catalyst demonstrated even higher activity, suggesting the formation of the Cu-ZnO interface.
"Our findings indicate that the encapsulation structure with S-1 effectively suppresses thermal aggregation of Cu particles, while simultaneously facilitating the rapid elimination of the water byproduct from the vicinity of the Cu-ZnO interface, thus enhancing methanol synthesis," remarked Tago.
Overall, this study demonstrates the effectiveness of the innovative encapsulation method for preparing highly active catalysts, offering a promising avenue for efficient methanol production from CO2.
More information: Ryokuto Kanomata et al, Development of Silicalite-1 encapsulated Cu-ZnO catalysts for methanol synthesis by CO2 hydrogenation, Chemical Engineering Journal (2024). DOI: 10.1016/j.cej.2024.149896
Journal information: Chemical Engineering Journal
Provided by Tokyo Institute of Technology