Strategy for evaluating energy performance of metal-organic-framework-based carbon dioxide adsorbents
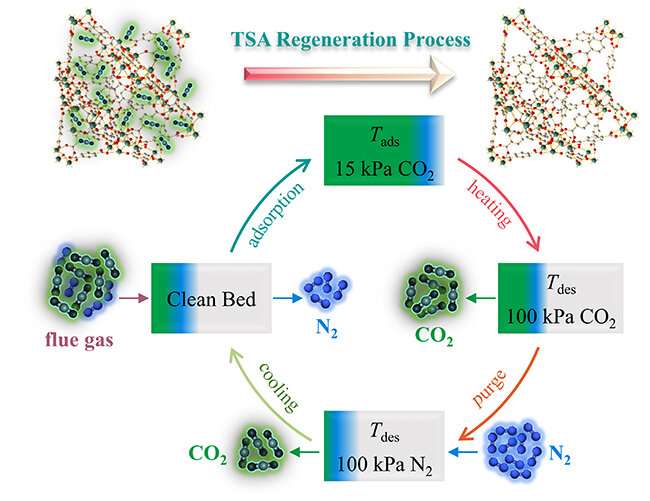
As promising carbon dioxide (CO2) adsorbents, metal-organic frameworks (MOFs) have attracted much attention in the field of carbon capture and storage (CCS).
In addition to the adsorption property, the energy performance related to the regeneration process is also a crucial factor when screening for suitable MOF adsorbents.
Recently, a research team led by Prof. Shi Quan from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. Han Wei from the Hong Kong University of Science and Technology, proposed an experimental strategy to investigate the energy performance of MOF adsorbents for CO2 capture in the temperature swing adsorption (TSA) process.
This study was published in the Chemical Engineering Journal on April 10.
The strategy is based on the combination of calorimetry and thermal analysis method. The researchers evaluated five well-characterized isomorphic zirconium-based MOFs using this strategy.
They analyzed the CO2 adsorption and desorption process using a thermogravimetric analysis instrument with a temperature-step program, and determined the desorption temperatures for these MOFs. The CO2 uptake and desorption heats of these MOFs were obtained from general isothermal adsorption measurements.
More importantly, the specific heat capacities of these MOFs were measured using a relaxation calorimeter, and their sensible heat values in the temperature swing range were calculated accordingly.
"The energy properties involved in the TSA process, including the regeneration energy, CO2 working capacity, and the corresponding parasitic energy, were efficiently and reliably evaluated," said Prof. Shi.
The results indicated that the sensible heat for heating the adsorbents from adsorption temperature to desorption temperature dominated the regeneration energy, and the parasitic energy was inversely proportional to the working capacity.
The proposed strategy contains few assumptions, and has sufficient resolution to distinguish small differences in energy efficiency-related properties of MOFs with similar structures and/or compositions.
"This new strategy may provide a feasible and effective experimental approach to study and evaluate the potential of adsorbents for CO2 capture," said Prof. Shi.
"This work can be a good reference for MOF adsorbent testing metrology development," commented one of the reviewers.
More information: Ji-Peng Luo et al, An experimental strategy for evaluating the energy performance of metal–organic framework-based carbon dioxide adsorbents, Chemical Engineering Journal (2022). DOI: 10.1016/j.cej.2022.136210
Provided by Chinese Academy of Sciences





















