This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
To study radioactive neptunium and plutonium, researchers establish a novel chemistry
Oxidation is the process where atoms lose electrons during a chemical reaction. Among the radioactive elements, neptunium and plutonium are much harder to oxidize than uranium.
To study these elements, scientists have designed donor ligands—molecules that contribute electron density to metal centers. This allows scientists to stabilize these metals as they become more electron-poor (in other words, reach higher oxidation states).
This moves their oxidation potentials (the energies at which it is possible to remove an electron) to a much more accessible range. This lets scientists study unusual complexes of cerium, uranium, and neptunium. In particular, it helps researchers examine how high oxidation states affect the structures and chemical behaviors of these elements.
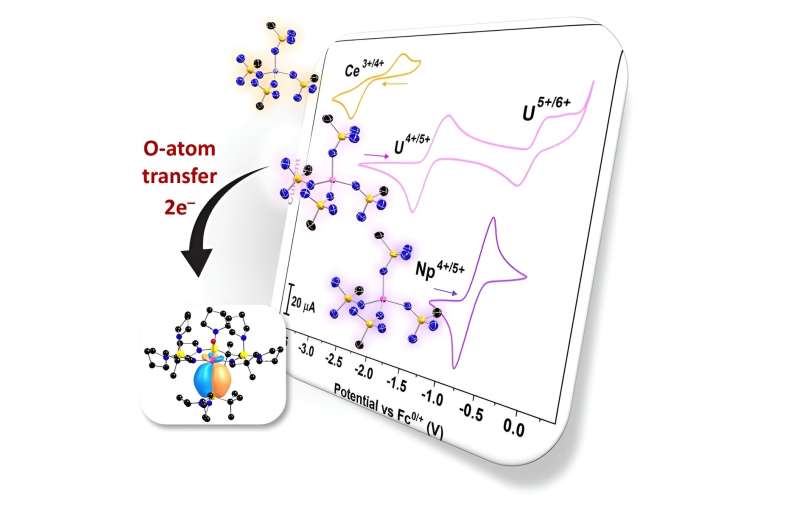
Accessing and studying the high oxidation states of uranium, neptunium, and plutonium complexes helps scientists understand their chemical reactivities—how easily they form new chemical compounds. It also helps scientists study their redox properties. These are the conditions under which the elements lose or gain electrons and the chemical products that result from these reactions.
These studies can shed light on how radioactive materials may behave in nuclear waste streams and waste storage. Additionally, the magnetic properties of these elements can affect the development of quantum information science and quantum materials.
However, these radioactive elements are hard to handle. This makes it difficult for scientists to develop their molecular chemistry. The ligands and electrochemical studies in the research described here will help address nuclear waste challenges.
This research developed a symmetry-breaking ligand that has enabled scientists to synthesize and conduct detailed characterization of non-aqueous uranium, neptunium, and plutonium complexes in high oxidation states. The findings are published in the journal Inorganic Chemistry and Angewandte Chemie International Edition.
Across the actinide series, the barrier to oxidation increases significantly after uranium, often making characterization of these complexes a challenge. The lower symmetry allows scientists to obtain better crystallographic data and conduct more thorough spectroscopic and theoretical examinations of the structure and electronic properties of these complexes. This ligand is strongly electron-donating and provides ample support to electron-poor, high-oxidation-state complexes, which would not otherwise persist.
It allows researchers to establish detailed synthetic strategies and non-aqueous electrochemical setups for the characterization of radioactive neptunium and plutonium complexes.
Electrochemical studies of cerium, uranium, and neptunium complexes show that this ligand has made the oxidation potentials of these species significantly more accessible. These oxidation potentials are corroborated by theory and inform the chemical reactivity and physical properties of these systems.
This sets the stage for the isolation and study of novel high-oxidation state neptunium and plutonium complexes.
More information: Julie E. Niklas et al, Ligand Control of Oxidation and Crystallographic Disorder in the Isolation of Hexavalent Uranium Mono-Oxo Complexes, Inorganic Chemistry (2023). DOI: 10.1021/acs.inorgchem.2c04056
Kaitlyn S. Otte et al, Divergent Stabilities of Tetravalent Cerium, Uranium, and Neptunium Imidophosphorane Complexes**, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202306580
Journal information: Angewandte Chemie International Edition , Inorganic Chemistry
Provided by US Department of Energy