This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study shows bacteria can survive on radioactive elements
Bacteria that feed on methanol are able to grow on certain rare earth elements as well as their radioactive relatives. These findings suggest a possible role for such bacteria in the decontamination of areas where actinides are spilled, or in the separation of lanthanides and actinides for analytical or preparative purposes, according to a study published in the journal Angewandte Chemie International Edition.
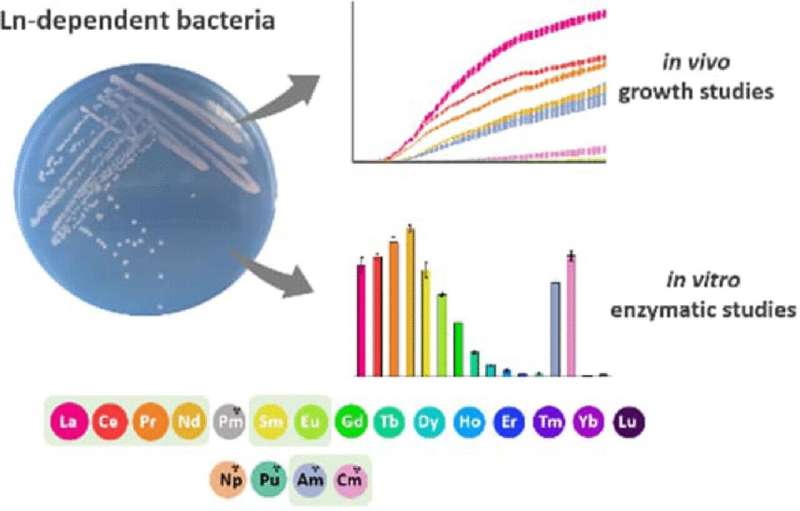
Lanthanides belong to the rare earth elements widely used in electronics and energy technologies. With one exception, they are not radioactive, they are abundant in the Earth's crust, and some lanthanides such as lanthanum and neodymium even play a crucial role in bacterial metabolism. Actinides, which include uranium and plutonium, are their heavy, radioactive counterparts, most of which are not found naturally on Earth. Now, Lena Daumann from Ludwig-Maximilians-University Munich, Germany, and her international interdisciplinary team have discovered that some actinides can replace essential lanthanides in the metabolism of methylotrophic bacteria.
Methylotrophic bacteria, so-called as they use methanol as their energy source, contain lanthanides in their methanol-oxidizing enzyme. The researchers observed that these bacteria could also take up actinide ions into this enzyme, using them for methanol metabolism in the same way as they would use lanthanides. This observation was particularly valid when the actinide ions were the same size and had the same stable +III oxidation states as the corresponding lanthanides. The bacteria even preferred americium and curium as actinides over some lanthanides when presented with a mixture of lanthanides and actinides, the researchers reported.
Americium and curium showed a stable +III oxidation state, which appeared to be particularly important. "When we used plutonium, which is known to have higher oxidation states, our bacteria did not grow, nor did the isolated enzymes work with it either," Daumann says. This knowledge will be useful for future applications. The team plans to explore the ability of the bacteria to extract actinides from radioactive waste or to separate out specific elements from mixtures.
Actinides such as plutonium, americium, and curium can be generated in nuclear reactors and are used in research, the nuclear industry, and many other technologies. Americium is even a source of ionizing radiation in commercial smoke detectors. However, actinides are highly radioactive and hazardous elements, which have to be handled with great care at special facilities. Spillages of radioactive substances are always of great concern. Dauman proposes "putting these bacteria to use to help clean up radioactive spills," as a possible future application.
More information: Helena Singer et al, Minor Actinides Can Replace Essential Lanthanides in Bacterial Life, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202303669
Journal information: Angewandte Chemie International Edition
Provided by Wiley