Very heavy elements deliver more electrons
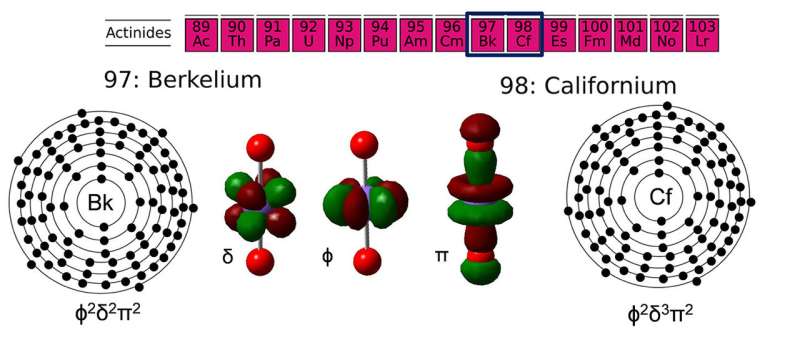
Actinides, a series of 15 radioactive elements, are vital to medicine, energy, and national defense. Scientists examined two exceedingly rare actinides, berkelium and californium. These elements are at the extreme end of what is possible to synthesize in more-than-atom amounts for chemical study. These elements are only available in tiniest amounts. The scientists showed that the elements can lose electrons to bond like lighter actinides. Specifically, they found the +5 oxidation state is much more stable than expected, and similar to the stability found for lighter actinides.
Due to the difficulty in studying these radioactive and rare elements, most of which have only been studied since the Manhattan Project, there is much to learn. Fundamental knowledge about the most basic, yet unknown, chemistry of the actinides, such as knowing better how two more actinides, berkelium and californium, form bonds at high oxidation states, could benefit environmental cleanup at nuclear production sites. Such knowledge could also aid in developing new nuclear fuels and their reprocessing. Further, this work resolves a longstanding uncertainty about actinides.
When forming bonds with oxygen or participating in reactions involving oxygen atom transfer, the early actinide elements, protactinium through curium, can attain the +5 oxidation state when bonded to two oxygen atoms. Scientists wanted to know if this oxidation state is important in bonding for berkelium and californium (the next two actinides in the series after plutonium, americium, and curium). The team synthesized positively charged berkelium and californium molecules that contain oxygen atoms within a mass spectrometer.
They created these molecules, BkO2+ and CfO2+, by transferring to the actinide monoxide cations a second oxygen atom from the common gas nitrogen dioxide. The team's high-level electronic structure calculations showed that the produced molecules are linear, a significant characteristic of positively charged pentavalent dioxide ions of the actinides. The dissociation energies to break the actinide-oxygen bonds, which are at least 73 kcal/mol, are surprisingly high. These first pentavalent berkelium and californium species reveal that the +5 oxidation state is possible farther into the actinide series than previously recognized, which is key to understanding the essential nature and chemistry of these elements.
More information: Phuong D. Dau et al. Remarkably High Stability of Late Actinide Dioxide Cations: Extending Chemistry to Pentavalent Berkelium and Californium, Chemistry - A European Journal (2017). DOI: 10.1002/chem.201704193
Journal information: Chemistry – A European Journal
Provided by US Department of Energy