May 4, 2018 report
Thorium-aluminum complex the first with an actinide element to donate electrons when bonding with a metal
A small team of researchers from the University of California, Lawrence Berkeley National Laboratory and LPCNO, Université de Toulouse, has developed a way to synthesize a thorium-aluminum complex with an actinide element to donate electrons when bonding with a metal. In their paper published in the journal Chemical Science, the group explains how they achieved the first-of-its-kind feat.
Thorium (Th) is a silver-colored radioactive metallic element. Like other metals, it is relatively hard, but bendable. It also has a high melting point and is very reactive—when exposed to air, it reacts and turns black. It is also considered to be unstable. It is currently used in certain welding applications and is being considered as a replacement material for uranium in some nuclear reactors.
As the researchers note, thorium's position on the periodic table is unique because of the reluctance of its 5f orbitals to engage in bonding, as occurs with other actinides. But it is also different chemically from other Lewis acidic transitions metals. In this new effort, the team set out to better understand the electronic structure of thorium by looking specifically at bimetallic complexes with metal-to-metal bonds. As part of that effort, they developed a way to synthesize Th–Al bimetallics using reactions between different materials. The resulting complexes are unique because the thorium atoms wound up in a +3 oxidation state. Notably, just 10 Th(III) complexes have ever been synthesized.
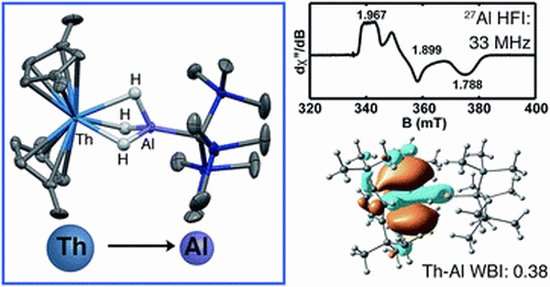
To synthesize the new Th(III) the researchers induced reactions between di-tert-butylcyclopentadienyl, supported by a Th(IV) dihalide, with an anionic aluminum hydride salt. The resultant material was then reduced, producing the new Th (III). To stabilize the new material, the researchers mated it with an alanate ligand.
To prove that that new material was in fact a Th(III), the researchers studied it using EPR spectroscopy, which revealed the shared electrons between the two atoms. They also conducted DFT calculations to show that the thorium had truly donated an election to the aluminum. The team suggests that their work may be of use to other chemists looking to use actinides as donors. They note also that the their experimental results could prove useful in the future as a way to make other actinides such as plutonium, reducing the need for other stabilizers.
More information: Alison B. Altman et al. Chemical structure and bonding in a thorium(iii)–aluminum heterobimetallic complex, Chemical Science (2018). DOI: 10.1039/C8SC01260A
Abstract
Thorium sits at a unique position on the periodic table. On one hand, there is little evidence that its 5f orbitals engage in bonding as they do in other early actinides; on the other hand, its chemistry is distinct from Lewis acidic transition metals. To gain insight into the underlying electronic structure of Th and develop trends across the actinide series, it is useful to study Th(III) and Th(II) systems with valence electrons that may engage in non-electrostatic metal–ligand interactions, although only a handful of such systems are known. To expand the range of low-valent compounds and gain deeper insight into Th electronic structure, we targeted actinide bimetallic complexes containing metal–metal bonds. Herein, we report the syntheses of Th–Al bimetallics from reactions between a di-tert-butylcyclopentadienyl supported Th(IV) dihalide (Cp‡2ThCl2) and an anionic aluminum hydride salt [K(H3AlC(SiMe3)3) (1)]. Reduction of the [Th(IV)](Cl)–[Al] product resulted in a [Th(III)]–[Al] complex [Cp‡2Th(μ-H3)AlC(SiMe3)3 (4)]. The U(III) analogue [Cp‡2U(μ-H3)AlC(SiMe3)3 (5)] could be synthesized directly from a U(III) halide starting material. Electron paramagnetic resonance studies on 4 demonstrate hyperfine interactions between the unpaired electron and the Al atom indicative of spin density delocalization from the Th metal center to the Al. Density functional theory and atom in molecules calculations confirmed the presence of An→Al interactions in 4 and 5, which represents the first examples of An→M interactions where the actinide behaves as an electron donor.
Journal information: Chemical Science
© 2018 Phys.org