Unusual structure, bonding, and properties may provide a new possibility for a californium borate
The heaviest element that exists in usable quantities on Earth, californium is surprisingly able to covalently bond with borate, dramatically altering the electronic characteristics of the californium ion, according to experimental and theoretical studies. These results challenge previously held views that heavy elements such as californium, plutonium, and americium are only able to possess purely ionic interactions.
This new paradigm in understanding may demonstrate how to further optimize the interactions between heavy elements, such as americium, curium, berkelium, and californium, and other elements to improve separating and recycling of used nuclear reactor fuel.
How the outer shells, or the valence orbitals, of the actinide elements (which are part of the heavy elements category) participate in bonding has been debated for decades. Recent experimental and computational investigations demonstrated the involvement of the outermost orbitals – 7p, 7s, 6d, and/or 5f – in bonding for early actinides such as uranium. However, structural and spectroscopic data, as well as theory, indicate a decrease in covalent bonding, where electrons are shared between two elements in their outermost valence orbitals, across the actinide series.
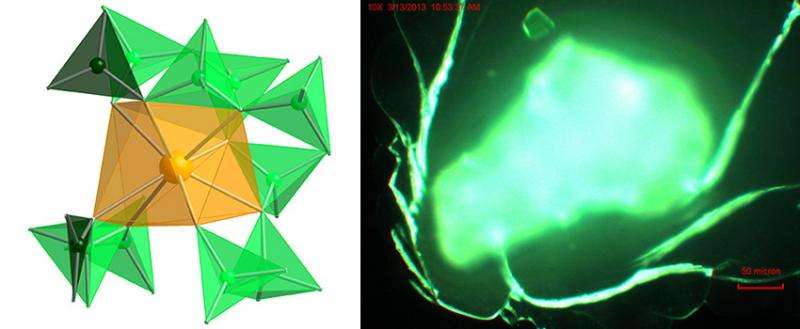
These late actinides, such as californium, are thought to possess highly ionic, lanthanide-like bonding where the attractive forces between the actinides and the ions surrounding them are purely electrostatic attraction between oppositely charged ions. To determine the chemical differences between californium and lanthanides, researchers "wrapped up" californium in an incredibly electron-rich environment using the ligand polyborate, which is both highly polarizable and can substantially rearrange upon complexation. The electrons from the ligands donated to the valence orbitals of californium, but surprisingly, researchers demonstrated that the 5f, 6d, 7s, and 7p orbitals are all involved in bonding and that large ligand-field effects are present. The synthetic, structural, and spectroscopic data are complemented by quantum mechanical calculations. The data suggest that californium chemistry is unusual and may be a link between the transition metals such as copper and platinum and the lanthanide and actinide elements.
More information: "Unusual structure, bonding and properties in a californium borate." Nature Chemistry 6, 387 (2014) DOI: 10.1038/nchem.1896
Journal information: Nature Chemistry
Provided by US Department of Energy