The remarkable variability of actinide tetrafluoride electronic structures
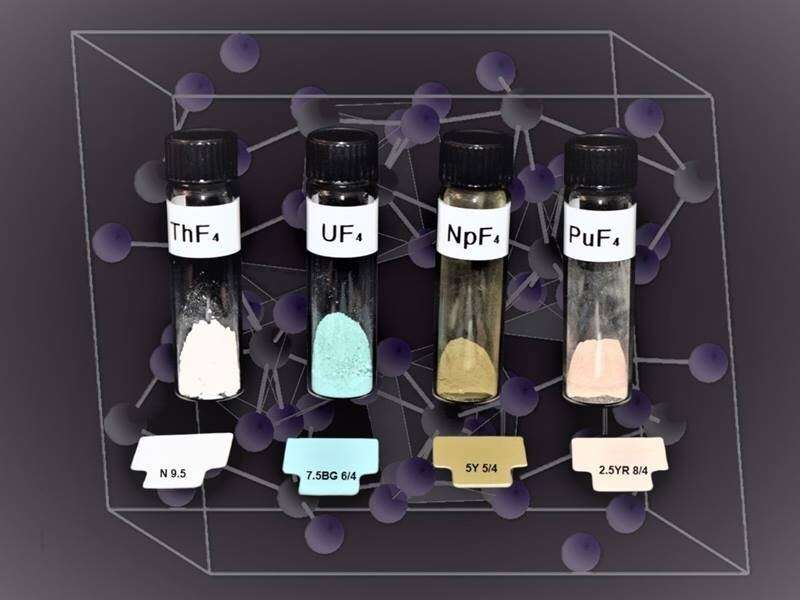
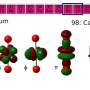
Scientists have synthesized tetrafluoride powders of four radioactive elements—thorium, uranium, neptunium, and plutonium. These four elements are actinides, a series of heavy and radioactive elements. Tetrafluoride powders are simply powders with four fluoride atoms per atom of actinide. In this new study, scientists probed the magnetic fields of these powders. This revealed remarkable variations in the electronic structures of the powders even though they have nearly identical crystal structures. These studies reveal the transition of valence electrons from itinerant to localized behavior across the actinide row of the periodic table; that is, for atoms of lighter elements of the row the electrons in the outer shell can be shared with neighbors, whereas for heavier elements electrons are confined to the atom. This research provides a basis for future studies of electronic configurations in other materials with similar crystal structures.
Actinide elements are key to the production of nuclear fuels and other energy technologies. Scientists therefore need accurate descriptions of the electronic structures of these elements. This will help researchers develop future nuclear fuels, superconductors, and other materials. This study presents a new way of mapping the distinctive evolution of electronic structure in actinide elements. Future studies will seek theoretical descriptions that relate the experimental observations to underlying structures.
Actinide elements such as uranium and plutonium play a leading role in energy and defense technologies. However, the scientific advances needed to realize the full potential of these technologies face complex theoretical issues in the analysis of heavy elements. Use of these advanced technologies also faces practical difficulties due to the special measures required to manage the radioactivity hazard. In this study, researchers synthesized a series of radioactive actinide tetrafluoride powders. These elements (thorium, uranium, neptunium, and plutonium) bridge the heavy and light end of the actinide row of the periodic table and display identical crystal structures for the tetrafluoride form. Scientists then probed the actinide tetrafluoride electronic structures by mapping the local fields using nuclear magnetic resonance (NMR) spectroscopy. Spectroscopic experiments were performed in the Radiochemical Processing Laboratory Radiological Nuclear Magnetic Resonance Facility, which houses instrumentation customized for the analysis of radioactive samples, including two NMR spectrometers and a broadband nuclear quadrupole resonance spectrometer. This work can be used a guide for future electron correlation studies of f-block elements.
More information: Eric D. Walter et al, Measurement of local magnetic fields in actinide tetrafluorides, The Journal of Chemical Physics (2021). DOI: 10.1063/5.0052323
Journal information: Journal of Chemical Physics
Provided by Pacific Northwest National Laboratory