New battery made of molten metals may offer low-cost, long-lasting storage for the grid
A novel rechargeable battery developed at MIT could one day play a critical role in the massive expansion of solar generation needed to mitigate climate change by midcentury. Designed to store energy on the electric grid, the high-capacity battery consists of molten metals that naturally separate to form two electrodes in layers on either side of the molten salt electrolyte between them. Tests with cells made of low-cost, Earth-abundant materials confirm that the liquid battery operates efficiently without losing significant capacity or mechanically degrading—common problems in today's batteries with solid electrodes. The MIT researchers have already demonstrated a simple, low-cost process for manufacturing prototypes of their battery, and future plans call for field tests on small-scale power grids that include intermittent generating sources such as solar and wind.
The ability to store large amounts of electricity and deliver it later when it's needed will be critical if intermittent renewable energy sources such as solar and wind are to be deployed at scales that help curtail climate change in the coming decades. Such large-scale storage would also make today's power grid more resilient and efficient, allowing operators to deliver quick supplies during outages and to meet temporary demand peaks without maintaining extra generating capacity that's expensive and rarely used.
A decade ago, the committee planning the new MIT Energy Initiative approached Donald Sadoway, MIT's John F. Elliott Professor of Materials Chemistry, to take on the challenge of grid-scale energy storage. At the time, MIT research focused on the lithium-ion battery—then a relatively new technology. The lithium-ion batteries being developed were small, lightweight, and short-lived—not a problem for mobile devices, which are typically upgraded every few years, but an issue for grid use.
A battery for the power grid had to be able to operate reliably for years. It could be large and stationary, but—most important—it had to be inexpensive. "The classic academic approach of inventing the coolest chemistry and then trying to reduce costs in the manufacturing stage wouldn't work," says Sadoway. "In the energy sector, you're competing against hydrocarbons, and they're deeply entrenched and heavily subsidized and tenacious." Making a dramatic shift in power production would require a different way of thinking about storage.
Sadoway therefore turned to a process he knew well: aluminum smelting. Aluminum smelting is a huge-scale, inexpensive process conducted inside electrochemical cells that operate reliably over long periods and produce metal at very low cost while consuming large amounts of electrical energy. Sadoway thought: "Could we run the smelter in reverse so it gives back its electricity?"
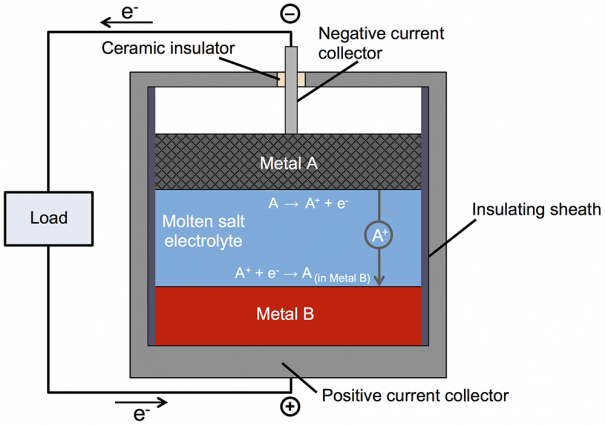
Subsequent investigation led to the liquid metal battery. Like a conventional battery, this one has top and bottom electrodes with an electrolyte between them (see Figure 1 in the slideshow above). During discharging and recharging, positively charged metallic ions travel from one electrode to the other through the electrolyte, and electrons make the same trip through an external circuit. In most batteries, the electrodes—and sometimes the electrolyte—are solid. But in Sadoway's battery, all three are liquid. The negative electrode—the top layer in the battery—is a low-density liquid metal that readily donates electrons. The positive electrode—the bottom layer—is a high-density liquid metal that's happy to accept those electrons. And the electrolyte—the middle layer—is a molten salt that transfers charged particles but won't mix with the materials above or below. Because of the differences in density and the immiscibility of the three materials, they naturally settle into three distinct layers and remain separate as the battery operates.
Benefits of going liquid
This novel approach provides a number of benefits. Because the components are liquid, the transfer of electrical charges and chemical constituents within each component and from one to another is ultrafast, permitting the rapid flow of large currents into and out of the battery. When the battery discharges, the top layer of molten metal gets thinner and the bottom one gets thicker. When it charges, the thicknesses reverse. There are no stresses involved, notes Sadoway. "The entire system is very pliable and just takes the shape of the container." While solid electrodes are prone to cracking and other forms of mechanical failure over time, liquid electrodes do not degrade with use.
Indeed, every time the battery is charged, ions from the top metal that have been deposited into the bottom layer are returned to the top layer, purifying the electrolyte in the process. All three components are reconstituted. In addition, because the components naturally self-segregate, there's no need for membranes or separators, which are subject to wear. The liquid battery should perform many charges and discharges without losing capacity or requiring maintenance or service. And the self-segregating nature of the liquid components could facilitate simpler, less-expensive manufacturing compared to conventional batteries.
Choice of materials
For Sadoway and then-graduate student David Bradwell MEng '06, PhD '11, the challenge was to choose the best materials for the new battery, particularly for its electrodes. Methods exist for predicting how solid metals will behave under defined conditions. But those methods "were of no value to us because we wanted to model the liquid state," says Sadoway—and nobody else was working in this area. So he had to draw on what he calls "informed intuition," based on his experience working in electrometallurgy and teaching a large freshman chemistry class.
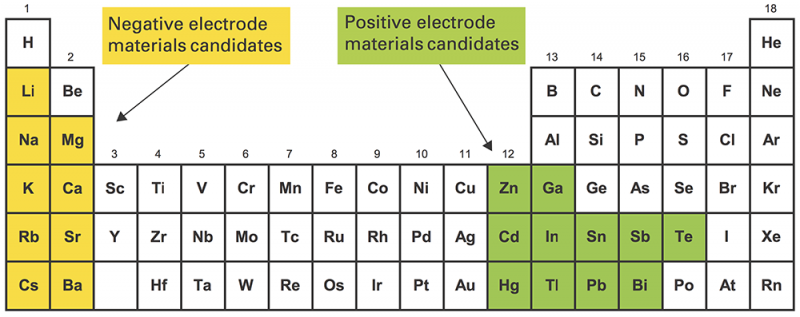
To keep costs down, Sadoway and Bradwell needed to use electrode materials that were earth-abundant, inexpensive, and long-lived. To achieve high voltage, they had to pair a strong electron donor with a strong electron acceptor. The top electrode (the electron donor) had to be low density, and the bottom electrode (the electron acceptor) high density. "Mercifully," says Sadoway, "the way the periodic table is laid out, the strong electropositive [donor] metals are low density, and the strong electronegative [acceptor] metals are high density" (see Figure 2 in the slideshow above). And finally, all the materials had to be liquid at practical temperatures.
As their first combination, Sadoway and Bradwell chose magnesium for the top electrode, antimony for the bottom electrode, and a salt mixture containing magnesium chloride for the electrolyte. They then built prototypes of their cell—and they worked. The three liquid components self-segregated, and the battery performed as they had predicted. Spurred by their success, in 2010 they, along with Luis Ortiz SB '96, PhD '00, also a former member of Sadoway's research group, founded a company—called initially the Liquid Metal Battery Corporation and later Ambri—to continue developing and scaling up the novel technology.
Not there yet
But there was a problem. To keep the components melted, the battery had to operate at 700 degrees Celsius (1,292 degrees Farenheit). Running that hot consumed some of the electrical output of the battery and increased the rate at which secondary components, such as the cell wall, would corrode and degrade. So Sadoway, Bradwell, and their colleagues at MIT continued the search for active materials.
Early results from the magnesium and antimony cell chemistry had clearly demonstrated the viability of the liquid metal battery concept; as a result, the on-campus research effort received more than $11 million from funders including Total and the U.S. Department of Energy's ARPA–E program. The influx of research dollars enabled Sadoway to grow the research team at MIT to nearly 20 graduate and undergraduate students and postdocs who were ready to take on the challenge.
Within months, the team began to churn out new chemistry options based on various materials with lower melting points. For example, in place of the antimony, they used lead, tin, bismuth, and alloys of similar metals; and in place of the magnesium, they used sodium, lithium, and alloys of magnesium with such metals as calcium. The researchers soon realized that they were not just searching for a new battery chemistry. Instead, they had discovered a new battery "platform" from which a multitude of potentially commercially viable cell technologies with a range of attributes could spawn.
New cell chemistries began to show significant reductions in operating temperature. Cells of sodium and bismuth operated at 560 degrees Celsius. Lithium and bismuth cells operated at 550 C. And a battery with a negative electrode of lithium and a positive electrode of an antimony-lead alloy operated at 450 C.
While working with the last combination, the researchers stumbled on an unexpected electrochemical phenomenon: They found that they could maintain the high cell voltage of their original pure antimony electrode with the new antimony-lead version—even when they made the composition as much as 80 percent lead in order to lower the melting temperature by hundreds of degrees.
"To our pleasant surprise, adding more lead to the antimony didn't decrease the voltage, and now we understand why," Sadoway says. "When lithium enters into an alloy of antimony and lead, the lithium preferentially reacts with the antimony because it's a tighter bond. So when the lithium [from the top electrode] enters the bottom electrode, it ignores the lead and bonds with the antimony."
That unexpected finding reminded them how little was known in this new field of research—and also suggested new cell chemistries to explore. For example, they recently assembled a proof-of-concept cell using a positive electrode of a lead-bismuth alloy, a negative electrode of sodium metal, and a novel electrolyte of a mixed hydroxide-halide. The cell operated at just 270 C—more than 400 C lower than the initial magnesium-antimony battery while maintaining the same novel cell design of three naturally separating liquid layers.
The role of the new technology
The liquid metal battery platform offers an unusual combination of features. In general, batteries are characterized by how much energy and how much power they can provide. (Energy is the total amount of work that can be done; power is how quickly work gets done.) In general, technologies do better on one measure than the other. For example, with capacitors, fast delivery is cheap, but abundant storage is expensive. With pumped hydropower, the opposite is true.
But for grid-scale storage, both capabilities are important—and the liquid metal battery can potentially do both. It can store a lot of energy (say, enough to last through a blackout) and deliver that energy quickly (for example, to meet demand instantly when a cloud passes in front of the sun). Unlike the lithium-ion battery, it should have a long lifetime; and unlike the lead-acid battery, it will not be degraded when being completely discharged. And while it now appears more expensive than pumped hydropower, the battery has no limitation on where it can be used. With pumped hydro, water is pumped uphill to a reservoir and then released through a turbine to generate power when it's needed. Installations therefore require both a hillside and a source of water. The liquid metal battery can be installed essentially anywhere. No need for a hill or water.
Bringing it to market
Ambri has now designed and built a manufacturing plant for the liquid metal battery in Marlborough, Massachusetts. As expected, manufacturing is straightforward: Just add the electrode metals plus the electrolyte salt to a steel container and heat the can to the specified operating temperature. The materials melt into neat liquid layers to form the electrodes and electrolyte. The cell manufacturing process has been developed and implemented and will undergo continuous improvement. The next step will involve automating the processes to aggregate many cells into a large-format battery including the power electronics.
Ambri has not been public about which liquid metal battery chemistry it is commercializing, but it does say that it has been working on the same chemistry for the past four years. According to Bradwell, Ambri scientists and engineers have built more than 2,500 liquid metal battery cells and have achieved thousands of charge-discharge cycles with negligible reduction in the amount of energy stored. Those demonstrations confirm Sadoway and Bradwell's initial thesis that an all-liquid battery would be poised to achieve better performance than solid-state alternatives and would be able to operate for decades.
Ambri researchers are now tackling one final engineering challenge: developing a low-cost, practical seal that will stop air from leaking into each individual cell, thus enabling years of high-temperature operation. Once the needed seals are developed and tested, battery production will begin. The researchers plan to deliver prototypes for field testing in several locations, including Hawaii, where sunshine is abundant but power generation still relies on burning expensive diesel fuel. One site is the Pearl Harbor naval base on Oahu. "It's unsettling that our military bases rely on the civilian power grid," says Sadoway. "If that grid goes down, the base must power up diesel generators to fill the gap. So the base can be without power for about 15 minutes, which is probably enough time for some major damage to be done." The new battery could play a key role in preventing such an outcome.
Meanwhile, back at the lab, the MIT researchers are continuing to explore other chemistries for the core of the liquid battery. Indeed, Sadoway says that his team has already developed an alternative design that offers even lower operating temperatures, more stored energy, lower cost, and a longer lifetime. Given the general lack of knowledge about the properties and potential uses of liquid metals, Sadoway believes there could still be major discoveries in the field. The results of their experiments "kicked open the doors to a whole bunch of other choices that we've made," says Sadoway. "It was really cool."
More information: David J. Bradwell et al. Magnesium–Antimony Liquid Metal Battery for Stationary Energy Storage, Journal of the American Chemical Society (2012). DOI: 10.1021/ja209759s
Hojong Kim et al. Liquid Metal Batteries: Past, Present, and Future, Chemical Reviews (2013). DOI: 10.1021/cr300205k
Xiaohui Ning et al. Self-healing Li–Bi liquid metal battery for grid-scale energy storage, Journal of Power Sources (2015). DOI: 10.1016/j.jpowsour.2014.10.173
Brian L. Spatocco et al. Low-Temperature Molten Salt Electrolytes for Membrane-Free Sodium Metal Batteries, Journal of The Electrochemical Society (2015). DOI: 10.1149/2.0441514jes
Kangli Wang et al. Lithium–antimony–lead liquid metal battery for grid-level energy storage, Nature (2014). DOI: 10.1038/nature13700
Journal information: Chemical Reviews , Journal of the American Chemical Society , Nature
Provided by Massachusetts Institute of Technology
This story is republished courtesy of MIT News (web.mit.edu/newsoffice/), a popular site that covers news about MIT research, innovation and teaching.




















