Compact and flexible thermal storage

Biogas plants, combined heat and power plants don't just generate electricity, they also produce heat. However, unlike the electricity they yield, the heat generally dissipates unused. A new technology is set to change this: It will allow the heat to be stored lossfree in the smallest of spaces for lengthy periods of time, for use as and when required.
There's a growing trend towards generating electricity from biogas. But these systems would be considerably more effective if better use could be made of the heat that is produced in the process. Roughly half of the total energy content of the fuel is released as heat, which typically dissipates into the atmosphere unused. Large quantities of heat likewise escape from combined heat and power plants, not to mention many industrial installations. The root of the problem lies in the fact that the heat is not generally used at the time it is generated – and options for storing it are limited. Traditionally, water tanks have been used for this purpose, but they can only absorb a finite quantity of heat. And of course, the heat can only be stored for short periods of time, because although the water tanks are insulated, the water gradually loses its heat to the surrounding atmosphere.
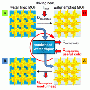
Working together with industrial partners such as ZeoSys GmbH in Berlin, scientists from the Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB in Stuttgart are currently developing a new type of thermal storage system. This new system can store three to four times the amount of heat that water can, so it only requires storage containers around a quarter the size of water tanks. Moreover, it is able to store the heat loss-free over lengthy periods of time and can even operate at temperatures well in excess of 100 degrees Celsius. The new system contains zeolite pellets, from the Greek zeō, meaning 'boil' and lithos, meaning 'stone'. Normally this material is used as an ion exchanger, for example to soften water. Because zeolites are porous, they have a huge surface area: A single gram of these pellets boasts a surface area of up to 1000 square meters. When the material comes into contact with water vapor, it binds the steam within its pores by means of a physicochemical reaction, which generates heat. The water is in reverse removed from the material by the application of heat and the energy is stored, but not as a result of the material becoming palpably warm – as when water tanks are used. What is stored is the potential to adsorb water and in the process release heat; the term 'sorptive thermal storage' is frequently used to describe these systems. And provided the dried zeolite material is prevented from coming into contact with water, it can store the heat for an unlimited amount of time.
Mobile test facility with a storage volume of 750 liters
Although the basic principle has been widely understood for some time, it had never before been translated into a broad-based technical application for storage systems. "We took the principle and confirmed it was technically feasible," says Mike Blicker, group manager, heat and sorption systems in the IGB. Initially, the researchers used a 1.5- and then a 15-liter reactor to demonstrate that the process really does work. Blicker explains: "First we developed the process engineering, then we looked around to see how we could physically implement the thermal storage principle – i.e. how a storage device has to be constructed, and at which locations heat exchangers, pumps and valves are needed." The institute's development partners were responsible for the material testing side of the project, investigating which of the various zeolites would be best suited for the purpose, how big the zeolite pellets needed to be, and whether or not the material would remain stable even after numerous storage cycles. They proved that heat could be stored and discharged many thousands of times without the system showing significant signs of wear and tear. The researchers subsequently up-scaled their operations to the current test facility, which has a storage volume of 750 liters and is mounted in a transportable container, along with all the additional equipment it requires. Its mobility allows the scientists to test the system in a variety of locations under realistic conditions.
The next stage of their work will be to reduce production costs, further optimize the system and adapt it for a variety of applications. Ultimately, the goal is to be able to store heat both in industrial installations and in small combined heat and power plants such as those used in larger residential buildings. To start with, priority will be given to industrial applications. "It would be ideal if we were able to devise a modular system that would allow us to construct each storage device to suit the individual requirement," says Blicker. The Fraunhofer researchers will be using a model system to demonstrate the principles of sorptive thermal storage at ACHEMA 2012 in Frankfurt from June 18 through 22 (Hall 9.2, Booth D64).
Provided by Fraunhofer-Gesellschaft