This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Developing new methods for targeted protein degradation
Northwestern Medicine scientists have developed a new, more precise method to target proteins implicated in certain types of cancer, according to a study published in Nature Chemical Biology.
Targeting proteins that contribute to cancer growth and treatment resistance can be accomplished with small molecule inhibitors, but those drugs only work on a subset of proteins, said Xiaoyu Zhang, Ph.D., assistant professor of Chemistry in the Weinberg College of Arts and Sciences and a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
"Traditional small-molecule drugs function by directly modulating protein activities," Zhang said. "The challenge is that some proteins have functional domains that are challenging to target with small molecules, such as transcription factors, splicing factors, et cetera. Another challenge arises with proteins that have multiple functional domains, which means small molecules that target just one of those domains may not fully inactivate protein functions."
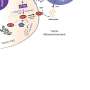
A promising alternative is to use small molecules to direct proteins to the cellular machinery for proteolytic degradation, resulting in complete protein removal.
"This targeted protein degradation (TPD) strategy can convert inactive protein-binding small molecules into active protein degraders and also operate catalytically, potentially lowering the required drug concentrations for therapeutic impact," Zhang said.
A growing number of proteins have been shown to be susceptible to ligand-induced protein degradation within cells. Most of these outcomes are mediated by two E3 ligases: CRBN and VHL. To fully realize the therapeutic potential of TPD, Zhang and his collaborators sought to identify additional E3 ligases to support this strategy.
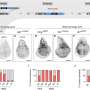
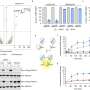
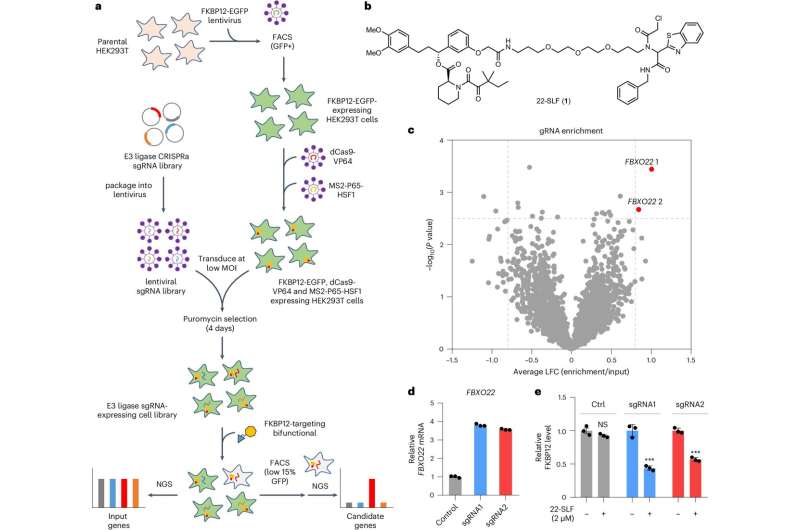
In the study, Zhang and his colleagues employed a CRISPR screen to discover human E3 ligases capable of supporting TPD. Investigators found that an E3 ligase FBXO22 could effectively mediate protein degradation within cultured cancer cells when stimulated with a class of drugs called PROTACs (proteolysis targeting chimeras).
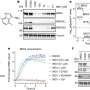
To further test their approach, investigators utilized their newly developed compound to hijack FBXO22 to degrade several other proteins associated with leukemia and breast cancer. The newly developed protein-targeting molecules were able to successfully mark proteins BRD4 and EML4-ALK for degradation within cancer cells, according to the study.
The results highlight a new approach for targeting proteins implicated in human disease, Zhang said.
"In many cancers, FBXO22 has increased expression," Zhang said. "Hijacking FBXO22 for TPD may present a promising approach for targeting cancer cells while potentially reducing toxicity in normal cells."
In future research, Zhang and his collaborators at the Lurie Cancer Center will continue to explore the use of TPD in different types of cancer.
"We can use this method to continue to discover new ligases that can support small molecule induced protein degradation," Zhang said. "We will be very interested in further exploring these ligases for their potential to degrade proteins in different disease contexts."
More information: Ananya A. Basu et al, A CRISPR activation screen identifies FBXO22 supporting targeted protein degradation, Nature Chemical Biology (2024). DOI: 10.1038/s41589-024-01655-9
Journal information: Nature Chemical Biology
Provided by Northwestern University