This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
ACE-ing protein detection in single cells
Since the 1950s, researchers have used a famous method invented by Wallace Coulter known as "flow cytometry" to characterize different types of immune cells in research studies and in blood samples from human individuals. This has enabled a much deeper understanding of immune cell development as well as new ways to assess human health and diagnose various blood cancers. Later, flow cytometry was also applied to other cell types.
In traditional flow cytometry, cell surface and intracellular proteins are detected with antibody molecules that are linked to fluorescent probes. However, while providing single-cell sensitivity, this method is limited in detecting multiple proteins by the number of fluorophores that can be clearly distinguished within the entire spectrum of fluorescent light.
The advent of "mass cytometry" in 2009 allowed the simultaneous quantification of 50 proteins in single cells, and a more fine-grained analysis of cells' identities and physiological states. In mass cytometry, antibodies are linked to non-radioactive isotopes of metal elements. These isotopes can be quantified in different channels of a mass cytometer instrument based on their mass.
However, mass cytometry, and its cousin "image mass cytometry" (IMC), which is used to visualize cell proteins in intact tissue slices, came at the cost of reduced sensitivity compared to flow cytometry and fluorescence microscopy.
Now, another 15 years later, a research collaboration led by the Wyss Institute at Harvard University and also including researchers from MIT and the University of Toronto has developed a method to significantly enhance the sensitivity of mass cytometry and IMC using DNA nanotechnology. Applying a new signal amplification technology called "Amplification by Cyclic Extension" (ACE) to DNA barcodes linked to antibodies, they were able to amplify protein signals produced by antibody-bound metal isotopes more than 500-fold, and to simultaneously and with high sensitivity detect more than 30 different proteins.
The new method enabled them to quantitively detect rare proteins, investigate complex biological tissue changes, and study how entire networks of interconnected proteins that regulate immune cell functions respond to stimulation and pathological conditions. Applied to IMC, ACE also allowed the identification of cell types and tissue compartments in histological sections, and changes in tissue organization related to the pathology of polycystic kidney disease.
The findings are reported in Nature Biotechnology.
"ACE helps to close a crucial gap in cytometric analysis: by enhancing the sensitivity of mass cytometry, it enables a single cell analysis platform that simultaneously achieves high sensitivity, high multiplexing, and high throughput. The opportunities it opens for investigating single cells in suspension and intact tissues with highly multiplexed and sensitive approaches can provide a much deeper understanding of normal and pathological biological processes," said Wyss Institute Core Faculty member Peng Yin, Ph.D., who led the study. Yin is also a Professor at Harvard Medical School (HMS)'s Department of Systems Biology.
More DNA, more metal isotopes, more sensitivity
Previously, Yin and his group at the Wyss Institute had developed multiple DNA-powered imaging technologies that can reveal the inner workings of cells with ultra-high resolution at the single molecule level, or by visualizing many distinct RNA and protein molecules in a single tissue slice. But the DNA structures that are created using these methods are not resilient enough to withstand the relatively harsh conditions used in mass cytometry.
"ACE solves current sensitivity problems of mass cytometry by allowing researchers to associate antibody molecules with substantially increased numbers of metal isotopes compared to conventional mass cytometry. This significantly facilitates the quantification of a broad range of low-abundance proteins, which has been challenging using previous single-cell approaches," said co-first author Xiao-Kang Lun, Ph.D., who is a Postdoctoral Fellow in Yin's group. Lun collaborated on the project with co-first author Kuanwei Sheng, Ph.D., who had initially developed ACE for other applications, including multiplexed imaging, and is also a Postdoctoral Fellow working with Yin.
"Inspired by our previous work on the Primer Extention Reaction for creating linear DNA concatamers (multiple copies of the same DNA sequence linked in series), and the PCR reaction which achieves amplification through synchronized thermal cycles, we invented ACE to synthesize linear concatamers in situ through thermal cycling in a controllable fashion," said Sheng.
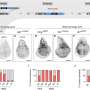
ACE creates a scaffold with multiple binding sites for short metal isotope-carrying "detector strands." In addition, by branching the synthesis of the scaffold strand, the researchers could further increase the method's sensitivity for the detection of rare proteins. Linear ACE on average provides a 13-fold signal amplification while branching ACE allows an initially unamplified signal to be increased more than 500-fold.
To stabilize the entire ACE sequence complex and keep it intact during mass cytometry analysis, they crosslinked the short double strands formed between the scaffold and the added detector strands with a chemical crosslinker.
"Following this recipe, we designed a panel with 33 distinguishable (orthologous) ACE sequences whose synthesis doesn't interfere with one another, and applied it to three entirely different types of analysis," said Sheng, who also is a Postdoctoral Fellow on Yin's team.
An ACE at work
The team first used ACE to investigate the transitions of epithelial cells into mesenchymal cells and back into epithelial cells again. Epithelial-mesenchymal transitions (EMTs) and mesenchymal-epithelial transitions (METs) occur during embryonic development but the former in particular is also re-enacted when tumors become invasive and metastatic.
By profiling in total 32 epithelial and mesenchymal markers, signaling molecules, and rare transcription factors in single mouse breast cancer cells multiple times during their 28-day transition from an epithelial to a mesenchymal state and back, and computationally parsing the results, they were able to shed new light on the two processes.
"ACE allowed us to profile levels of low-abundance transcription factors simultaneously with markers reflecting cellular physiological and signaling states in single cells. This led to a more refined picture of how molecular programs in EMT and MET are driven by increasing and decreasing amounts of key transcription factors, including Zeb-1 and Snail/Slug," said Sheng.
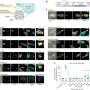
In their second example, they zoomed into the inner workings of single T cells. The stimulation of T-cell receptor (TCR) molecules on their surface results in the activation of a complex network of intracellular signaling proteins. Analyzing these signaling responses at single-cell resolution has been difficult, also due to T cells' small size. Individual proteins of this network are activated by phosphate residues that are attached to them by other network proteins generally known as kinases.
Many of these activated network proteins go on to phosphorylate other proteins of the network. This ultimately leads to changes in T-cell behavior, for example, toward pathogens or cancer cells. The researchers applied ACE to a panel of 30 antibodies that specifically bound to phosphorylated motifs in TCR-network proteins with functions in stress, inflammation, cell proliferation and other responses.
"Using ACE-enhanced mass cytometry analysis, we captured quantitative snapshots of the dynamically changing TCR network in individual primary human T cells. This allowed us to study the single-cell variations in the timing and duration of specific T-cell activation events and to reveal how the network is activated from its ground state by extracellular cues," said Lun.
The team used the same ACE-enhanced antibody panel to investigate a phenomenon known as "injury-induced T-cell paralysis." T cells experiencing injury in their environment, such as tissue injuries caused in major surgical procedures, often become immunosuppressive.
To start to understand how the TCR network causes this, Yin's group collaborated with co-author Michael Yaffe, M.D., Ph.D., who is the David H. Koch Professor of Science and Professor of Biology and Biological Engineering at MIT and has a strong interest in how the microenvironment surrounding sites of tissue injury suppresses the immune system. Yaffe provided the team with samples of "postoperative drainage fluid" (POF) that were obtained from patients undergoing surgery. Stimulating T cells with POFs as well as their TCRs enabled the researchers to isolate distinct network changes that cause single T cells to stop dividing and become exhausted.
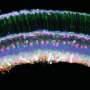
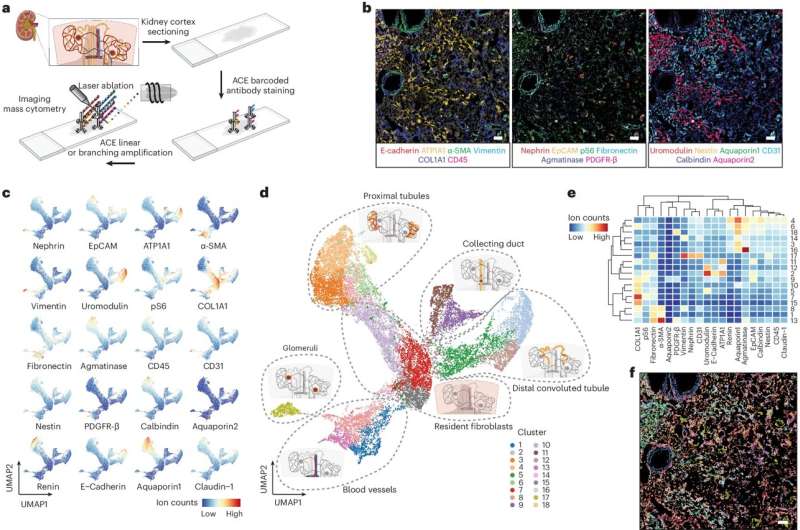
Finally, they investigated the utility of ACE also for spatial analysis of proteins in tissue sections using IMC by focusing on the human kidney. Kidney tissue is difficult to analyze by fluorescence microscopy because of its strong autofluorescence, and by traditional IMC because it lacks sensitivity.
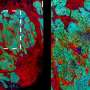
The researchers developed a panel of 20 ACE-enhanced antibodies for various kidney markers and used it to examine sections of the renal cortex derived from a patient with polycystic kidney disease. This approach, in which they collaborated with co-author Hartland Jackson, Ph.D., Professor at the University of Toronto, Canada and expert in multiplexed imaging, allowed them to identify the different cell types and their organization within the kidney's proximal and distal tubules, collecting ducts, and blood-filtering glomeruli.
"We discovered new disease-specific features of cell and tissue organization and found that the stem cell marker Nestin, which is also associated with kidney disorders, was expressed very heterogeneously across glomeruli," said Lun. "This could mean that different parts of the tissue could be simultaneously going through different pathological stages."
"This new mass cytometry approach developed by Peng Yin's team and their collaborators once again shows the power of leveraging DNA nanotechnology to turbocharge an existing technique that is highly relevant for clinical care, and to bring it to a much higher level of sensitivity and specificity. This relatively simple method will lead to entirely new insights into cell, tissue, and organ function, both during health and disease," said co-senior author and Wyss Founding Director Donald Ingber, M.D., Ph.D., whose group provided critical expertise on stimulating T cells. He is also the Judah Folkman Professor of Vascular Biology at HMS and Boston Children's Hospital, and the Hansjörg Wyss Professor of Bioinspired Engineering at the Harvard John A. Paulson School of Engineering and Applied Sciences.
Other authors on the study are Xueyang Yu, Ching Yeung Lam, Gokul Gowri, Matthew Serrata, Yunhao Zhai, Hanquan Su, Jingyi Luan, and Youngeun Kim.
More information: Xiao-Kang Lun et al, Signal amplification by cyclic extension enables high-sensitivity single-cell mass cytometry, Nature Biotechnology (2024). DOI: 10.1038/s41587-024-02316-x
Journal information: Nature Biotechnology
Provided by Harvard University