This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
How bacteria attach their cloaks of invisibility to immune defenses
Bacteria have different strategies for protecting themselves. Some bacterial pathogens surround themselves with a shell made of many sugar chains that lie close together, also known as capsular polymers. This protects the bacteria from drying out and physical stress. In addition, the capsule makes the pathogens invisible to our body's own defenses, so to speak, and helps them to survive in the body.
Preventing the capsule from being built would weaken the bacteria significantly. Enzymes that build such capsules are therefore potential targets for drugs and valuable biotechnological tools for the production of vaccines. Despite their importance, it is still unknown how the capsule polymers—which differ greatly depending on the type of bacteria—are attached to the bacterial membrane.
'Anchor chain' and enzymes decoded
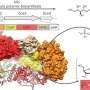
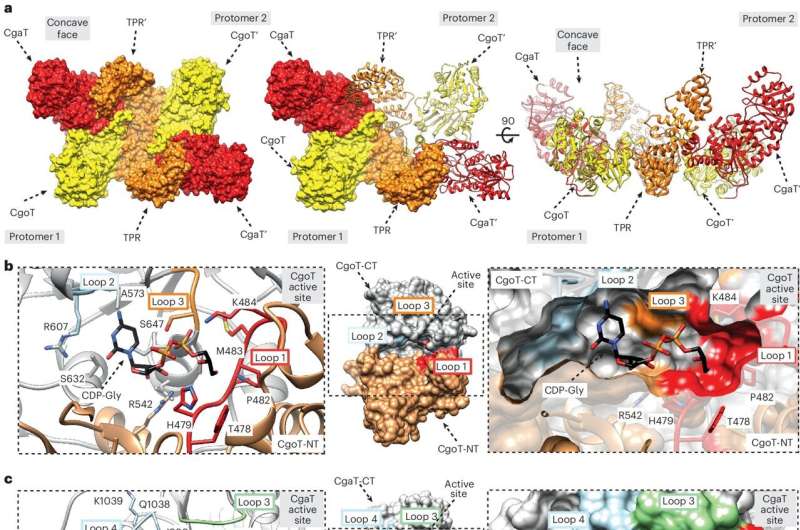
A fatty acid molecule is located in the membrane itself as an "anchor." The intermediate piece, i.e. the linker connecting the anchor and the capsule, has now been identified by an international team led by Dr. Timm Fiebig, head of the "Microbial Glycobiochemistry and Vaccine Development" working group at the Institute of Clinical Biochemistry at the Hannover Medical School (MHH). The researchers have not only succeeded in precisely describing this so-called linker in a larger group of bacterial pathogens, but also in characterizing the enzymes called transition transferases that produce the linker.
With the initial description, these are now available as potential target structures for the development of antibacterial agents and as synthesis tools for vaccine development. The work has been published in the journal Nature Chemical Biology.
Capsule-forming enzymes extend the chain of links
The so-called capsular polymerases, which produce the different polysaccharide capsules, also play an important role in the formation of the chain.
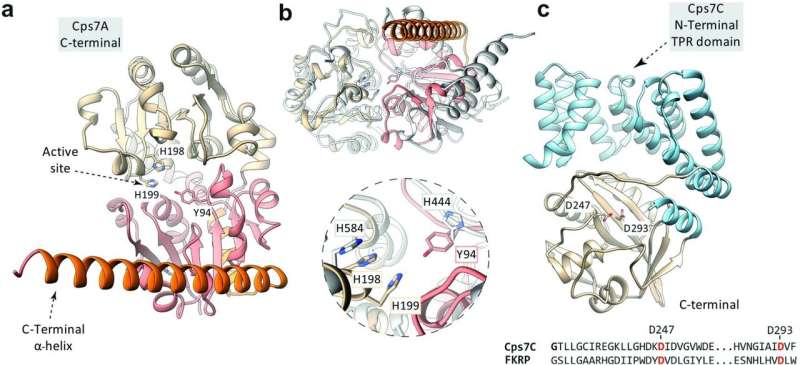
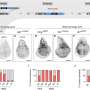
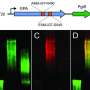
"The polymerase recognizes the linker and can extend it," explains Dr. Fiebig. The researchers were also able to clarify this step in the capsular biosynthetic pathway. Using a special chromatography device, they were able to purify the enzymes and the linker, examine their structure and reproduce the capsule synthesis in a test tube.
"This showed that the transition transferases stimulate the capsule polymerase to form particularly long sugar chains, which presumably protect the bacterium even more effectively," the biochemist notes. The Fiebig group has already been able to demonstrate how the capsular polymerases build up the bacterial capsule in earlier studies, including for the bacterium Haemophilus influenzae type b (Hib), which causes infections of the upper and lower respiratory tract, but also more serious diseases such as middle ear infection, meningitis or blood poisoning.
Starting point for new antibacterial drugs
In addition to the potential biotechnological use of the enzymes, the work is also important for basic research into the production of capsules.
"On the one hand, we were able to show that transition transferases occur in conserved regions of the bacterial genome, i.e. their genes are always found in the same place in the genome, regardless of the bacterial species," explains Dr. Christa Litschko, a scientist at the institute and the first author of the study.
"On the other hand, we have discovered that the linker is structurally different from the capsular polymer, contrary to previous assumptions." These observations will help to find further candidates of this enzyme class that create connections between the outer membrane of the bacterium and its capsule.
Since different types of bacteria can use the same method to produce the anchor chain, this could be a starting point for drugs that, like antibiotics, can be used against several strains of bacteria. In their research, the scientists have found similarities in the design and construction of the linker in various pathogens that can cause meningitis and urinary tract infections, for example.
"By inactivating the enzymes that build the linker, we could prevent the attachment and formation of the capsule shell, leaving the bacterium defenseless against attack by the immune system," emphasizes Dr. Fiebig. "However, a lot of research is still needed before we get there."
More information: Christa Litschko et al, Transition transferases prime bacterial capsule polymerization, Nature Chemical Biology (2024). DOI: 10.1038/s41589-024-01664-8
Journal information: Nature Chemical Biology
Provided by Hannover Medical School