This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Breakthrough in nonoxidative coupling of methane: Direct conversion to propylene in low temperature
Nonoxidative coupling of methane (NOCM) exhibits promising prospect in that it affords value-added hydrocarbons and hydrogen with high atom economy. However, the challenge remains in methane's direct, selective conversion to more valuable hydrocarbons like olefins.
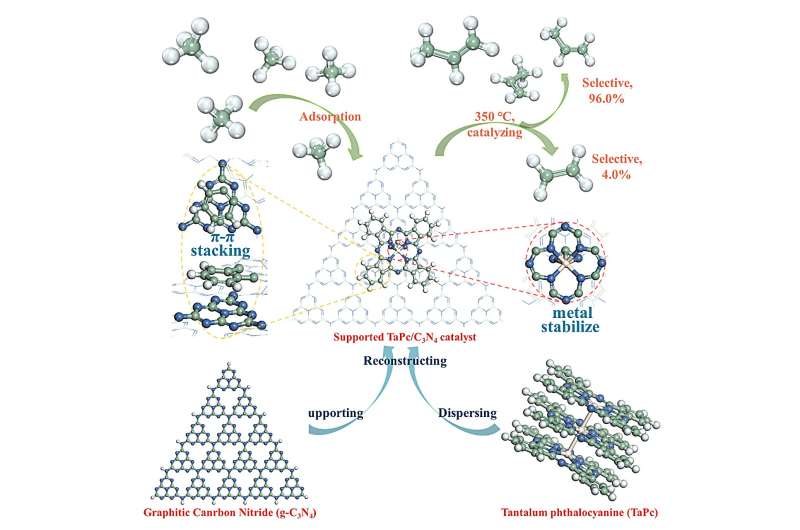
The current work presents a catalyst with well-dispersed Ta atoms anchored by g-C3N4 supported phthalocyanine. Such a catalyst is able to convert methane selectively to ethylene and propylene at a relatively low temperature (350oC).
The conception of the active center and construction of the catalyst have been described, and the origins of the catalytic performance are discussed. The relevant achievements were published in Research under the title of "Direct Conversion of Methane to Propylene."
The growing availability of low-cost and abundantly sourced natural gas leads to increased interest in its conversion to value-added chemicals. Natural gas is composed of dominantly small hydrocarbons, with methane typically taking a volumetric fraction of about 70–90%. Nowadays, great efforts have been made to convert methane into more useful chemicals via direct or indirect routes.
The indirect route involving the methane reforming and Fischer-Tropsch processes plays a crucial role in industry, as it affords one of the most important classes of chemicals—olefins. However, such a two-step conversion sequence wastes a considerable part of methane molecules by unavoidably producing useless CO2 and H2O. By contrast, direct methane conversion shortens the reaction paths and utilizes more proportion of methane.
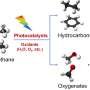
Generally, there are two major routes for the direct conversion of methane to light olefins, i.e., oxidative coupling of methane (OCM) and nonoxidative coupling of methane (NOCM). The OCM process uses an oxidant to overcome the thermodynamic restrictions and make the reaction exothermic.
However, byproducts like CO2 and H2O are still unavoidable, decreasing the atom economy. Since the 1990s, numerous efforts have been made to produce hydrocarbons through NOCM processes. However, the challenges with high reaction temperatures and cooking remain.
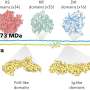
To weaken the Ta-C bond, an electron-rich Ta center is required. This is supported by a previous report on the highly efficient thermal activation of methane by [TaN]+ in the gas phase. Thus, a Ta-N unit is generally preferable for methane conversion, and a Ta-N4 center may be built in the condensed phase. Herein, a catalyst was prepared with Ta-N4 center as anchored in phthalocyanine, and it is supported by g-C3N4 in order to stabilize the mental center and disperse Ta phthalocyanine (TaPc) via π-π stacking.
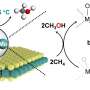
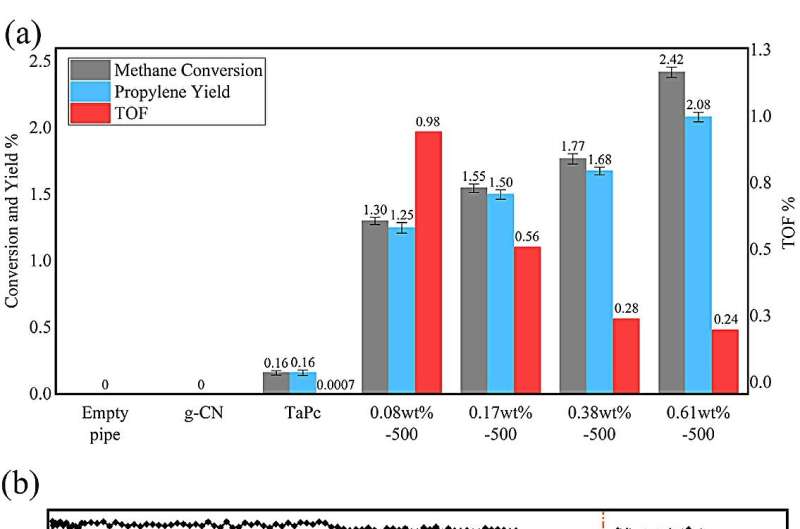
Through activation under hydrogen flow, the so-prepared TaPc/C3N4 catalysts exhibit surprisingly high activity toward methane conversion. The 0.08wt% Ta sample gives the highest TOF with the valve of 0.99 s-1 at 350℃.
At this condition, the selectivity of propylene is up to 96.0%, corresponding to 4.0% ethylene. Further, in the lifetime test, the 0.08wt% Ta sample affords a long single-run lifetime of > 300 h at 350℃ with stable conversion of methane; after reactivation, it still lasts for > 120 h.
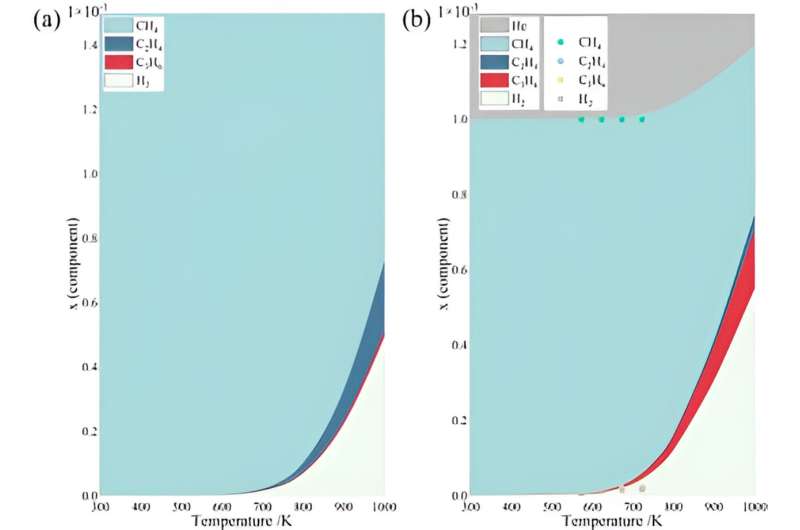
According to the Gibbs-Helmholtz formula, the thermodynamic limit of this work is evaluated. Considering a coupling consecutive reaction, it shows that the experimentally observed conversion almost approaches its thermodynamic limit at a lower temperature.
Also, with the same total pressure, inert gas actually plays a dilution role in the system. According to Le Chatelier's principle, with a positive stoichiometric coefficient, the NOCM process is favored with higher conversion. In addition, the presence of the inert gas allows for deeper condensation of the system, thus facilitating propylene production. Alternatively, the possibility for NOCM proceeding as parallel processes was also considered, while inconsistence was found in kinetic modeling.
Further, quantum chemical calculations were performed to probe the origins of the performance of the TaPc/C3N4 catalyst. A model with TaPc(C32N8H16Ta) supported on C3N4 (C90N123H15) via π-π stacking was thus built.
The Semiempirical Extended Tight-Binding (xTB) Computation Method was employed in conjunction with the gau_xtb code to perform feasible calculations on such a large model. Here, we focused on why ethylene and propylene correspond to the major product.
According to the calculation, the bridge N-CR2-Ta (R = H, CH3) structures serve as the key intermediates, which either enable the carbon-chain propagation or, alternatively, isomerize to release olefin molecules. Considering the vital role the Ta atom plays in the transformation that it serves as the courier to deliver both carbon and hydrogen, most likely, here, the relativistic effects induced strong Ta-C/H interaction functions once again.
Future efforts may focus on how to uniformly load higher content of well-dispersed Ta on a carbon-based material and how to enhance the chemical stability of the Ta-N4 structure. Further, the relativistic effects exert a similar influence on the gas and condensed phases, which encourages us to continue the gas-phase-guided construction of high-performance catalysts.
Indeed, gas-phase studies allow us to correlate various structural/electronic features with the performance of the active center, while the major difficulty is still on how these favorable features are replicated in bulk systems. As to the direct conversion of methane to higher hydrocarbons, most likely, a 5d-element centered structure is necessary so as to bind the carbonide intermediates strongly for further propagation of the carbon chain.
More information: Yunpeng Hou et al, Direct Conversion of Methane to Propylene, Research (2023). DOI: 10.34133/research.0218
Journal information: Research
Provided by Research