This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Team reviews photocatalysis for methane conversion to high-value products
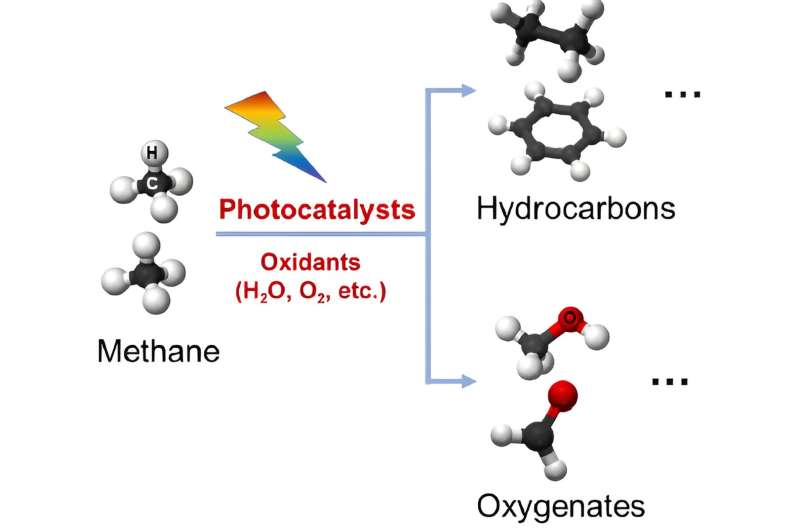
A research team has published a review paper summarizing the recent progress in methane conversion using photocatalysis. Photocatalysis, the process of using light to accelerate a chemical reaction, offers a promising and green technology for methane conversion to high-value fuels and chemicals. In their paper, the team details the advances using photocatalysts for methane conversion and outlines the challenges that remain.
The team published their findings in a review paper in Carbon Future.
The current commercial route for large-scale transformation of methane involves the indirect conversion via synthesis gas. Because methane is stable and inert, activating and converting the molecule is extremely challenging. The current commercial process involves high energy consumption and carbon dioxide release.
"The shift to methane conversion by net-zero carbon dioxide emissions is a critical task for both scientific research and industry, as it will produce high-value chemicals without potentially catastrophic consequences," said Junwang Tang, a professor at University College London. Bypassing the synthesis gas route and using photocatalysis to convert methane to valuable hydrocarbons and oxygenates offers a green alternative as photocatalysis is superior in activating and converting inert methane to hydrocarbons and oxygenates under mild conditions.
Methane is a relatively clean and low-cost fuel. It is found abundantly in shale gas and methane hydrate worldwide. Methane can also be naturally generated from biosystems and produced artificially from biomass. This abundance makes it appealing for use in power generation and residential heating. Among the conventional fuels, methane has a high caloric value and is easily transported using pipes. However, as a greenhouse gas, methane has a higher global warming potence than carbon dioxide.
Over a 100-year period, methane warms the Earth at least 25 times as much as the equivalent mass of carbon dioxide. In industry, methane is widely used as a feedstock, producing chemicals, fertilizer, and hydrogen. In 2021, industry in U.S. used about 33 percent of the total natural gas. Natural gas is also the energy source for about 33 percent of U.S. industry.
So methane is currently used not only as a fuel but also as a fundamental chemical source. Society's continued reliance on methane to meet growing energy needs leads to negative consequences for the environment. Scientists need to develop technology for methane conversion to more useful, high-value carbon chemicals by a low or zero carbon process.
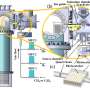
Unlike thermal catalysis, which involves high-reaction temperatures, greater than 400 °C, or expensive oxidants, photocatalysis uses sunlight or artificial light sources as the only energy input. Photocatalysis can selectively oxidize methane to C2+ hydrocarbons or oxygenates using low-cost, environmentally friendly oxidants, such as water and oxygen at low temperatures.
The team notes that photocatalysis is not without drawbacks at this time. These include its low yield rate and limited conversion to more valuable products. "Additionally, greater attention should be paid to higher value products, which remains a significant challenge in this field," said Tang.
Looking ahead, the team will focus their efforts on increasing the methane conversion rate and converting methane into more valuable products by designing highly efficient photocatalysts with active reaction sites, as well as optimizing the reaction system. "Our ultimate goal is to produce the seven most basic chemicals by photocatalytic methane conversion," said Tang.
If the team can achieve this goal, a considerable portion of chemicals like oxygenates, hydrocarbons and polymers could be obtained through the low-carbon process of photocatalytic methane conversion. "This could significantly reduce energy consumption and carbon dioxide emissions during the production of the major chemicals," said Tang.
More information: Youxun Xu et al, Photocatalytic methane conversion to high-value chemicals, Carbon Future (2023). DOI: 10.26599/CF.2023.9200004
Provided by Tsinghua University Press