A new strategy for enhancing hydrogen peroxide utilization in photocatalytic reactions
Recently, a research team led by Prof. Huang Weixin from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS) realized the production of oxygenated chemicals from methane and H2O2 with oxide semiconductor as photocatalyst, revealed a new strategy for improving H2O2 utilization and photocatalytic conversion efficiency from methane to oxygenates, and studied the photocatalytic activity and selectivity by tuning TiO2 facets.
This work was published in Nature Communications.
Methane has been increasingly considered as a possible carbon resource, but it is a major challenge to convert methane to value-added chemicals with both high activity and selectivity.
H2O2 is a common oxidant that could photocatalytically convert liquid-phase methane to value-added chemicals. It reacts with photogenerated electrons and produce ·OH radicals or ·OOH radicals, which is an effective activation method. Unfortunately, H2O2 is subject to side reactions with photogenerated holes and tends to convert itself to O2, reducing its utilization.
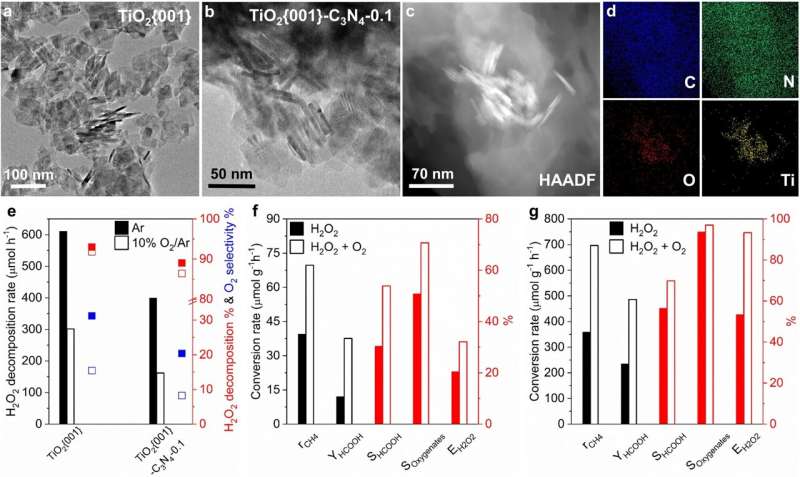
In this study, the researchers aerated O2 into the semiconductor photocatalytic reaction system containing methane and H2O2, and found that H2O2 utilization was enhanced. They discovered that the adsorption of O2 onto the surface of TiO2 inhibited the adsorption of H2O2, which suppressed side reactions.
In addition, the researchers observed that photocatalytic activity and selectivity was determined by TiO2 facets. In virtue of theoretical calculation, they discovered that it was the desorption energy of different products on different TiO2 facets that accounted for the different catalytic selectivity. For example, CH3OOH had low desorption energy on TiO2{101}, thus it was the only liquid-phase product of TiO2{101}-C3N4 composite.
This work offered a new strategy for improving H2O2 utilization and conversion efficiency in the photocatalytic conversion from methane to oxygenates. It also manifested the role that the catalyst facets played during photocatalytic reactions.
More information: Xiao Sun et al, Molecular oxygen enhances H2O2 utilization for the photocatalytic conversion of methane to liquid-phase oxygenates, Nature Communications (2022). DOI: 10.1038/s41467-022-34563-4
Journal information: Nature Communications
Provided by Chinese Academy of Sciences