This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers realize direct conversion of methane with oxygen at room temperature
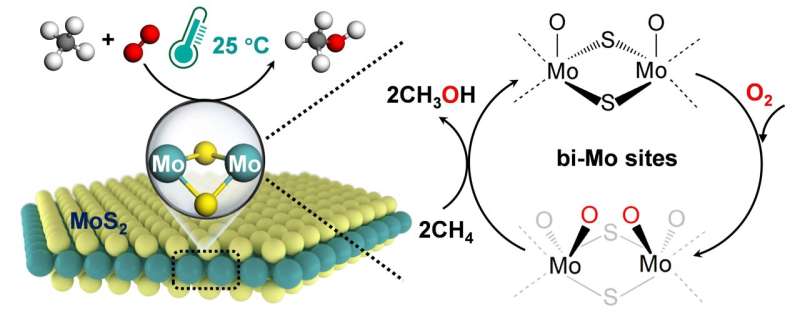
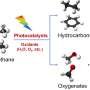
Direct conversion of methane (CH4) to high-value-added chemicals at room temperature, by directly using abundant and low-cost molecular oxygen (O2) as an oxidant, is an ideal route for CH4 utilization. But it remains a challenge due to the chemical inertness of methane and low activity of O2.
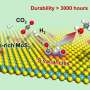
Recently, a research group led by Prof. Deng Dehui and Assoc. Prof. Yu Liang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) realized direct CH4 conversion to C1 oxygenates (CH3OH, HOCH2OH and HCOOH) with O2 at room temperature (25℃) over an edge-rich MoS2 catalyst. The study was published in Nature Catalysis on Sept. 21.
Catalytic conversion of methane to high-value-added chemicals is a tough problem due to the low polarization rate and high C-H bond energy (439 kJ mol-1) of methane.
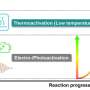
Typical catalytic conversion of CH4 usually operates at high temperatures (over 600℃), or in the aid of strong oxidants (such as fuming sulfuric acid) or external fields (such as plasma). Nevertheless, such harsh reaction easily leads to excessive conversion of the target product, such as overoxidation to CO2.
Direct conversion of CH4 and O2 at low temperatures or even at room temperature is an appealing strategy for CH4 conversion. However, it is challenging due to the difficulty in continuous formation of active oxygen species under mild conditions for C-H activation.
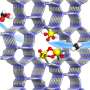
In-situ characterizations and theoretical calculations demonstrated that the unique binuclear molybdenum (bi-Mo) site of sulfur vacancies at the MoS2 edge was able to directly dissociate O2 to form O=Mo=O* active species at 25℃, which could activate the C-H bond of CH4 and thereby driving the catalytic conversion of CH4 to C1 oxygenates via CH3O* intermediates at room temperature.
In this study, the researchers achieved CH4 conversion of up to 4.2% with a high selectivity of over 99% for the C1 oxygenates for CH4 conversion with O2 at room temperature.
More information: Jun Mao et al, Direct conversion of methane with O2 at room temperature over edge-rich MoS2, Nature Catalysis (2023). DOI: 10.1038/s41929-023-01030-2
Journal information: Nature Catalysis
Provided by Chinese Academy of Sciences