This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New ligand-guided technique enhances drug development
Achieving a level of precision to create complex medicines and materials with extreme accuracy is a longstanding goal of scientists and pharmaceutical companies. If achieved, that precision could lead to the development of more effective drug treatments.
Now, scientists at the University of Rochester—led by Shauna Paradine, an assistant professor in the Department of Chemistry—have developed a groundbreaking method to yield products by using a chemical "helper" to guide reactions in an extremely precise manner. The research, published in Nature Communications, opens the door for more efficient drug development while also providing fundamental advancements in the creation of new materials.
Overcoming molecular bias
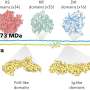
In chemical reactions, there are often multiple ways that two molecules can come together. It is a challenge, however, for scientists to control exactly how these interactions occur. Sometimes molecules have a bias—known as a substrate bias—in the way they interact, preferring to react or combine in a specific way.
"If you can overcome that bias, you have the ability to access configurations of your product molecules that you could not otherwise access," Paradine says.
What is a ligand?
A ligand is an atom, ion, or molecule that binds to a central metal atom or ion. The nature of the ligands and the central metal atom can influence the properties and reactivity of the resulting complex.
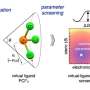
Paradine and her team discovered a method to overcome substrate bias using a ligand, making the reaction more precise. Specifically, the ligand was able to switch the direction in which the molecules react, without altering any other part of the chemical process.
The team tested many phosphorus-based ligands, which are commonly used for reactions involving palladium metal. Only one of them was able to switch the selectivity of the reaction.
"It was very much like picking out a needle from a haystack," Paradine says.
Transforming ligand chemistry—and drug discovery
Although the researchers used a specific ligand in one particular reaction in this study, they applied the data to create a predictive model to understand the selectivity they were observing in reactions. The model helps determine how different ligands affect a reaction, paving the way for using a ligand-centered approach in other types of chemical reactions.
The research has important implications in drug discovery and development.
"The products we're making contain core structures that are often found in biologically active molecules," Paradine says. "In drug discovery, it's really important to be able to finely tune a molecule to optimize its various properties."
In pharmaceutical research, altering and optimizing a molecule's properties typically requires a complex amount of work. By simply changing a ligand, scientists can now get different configurations of a molecule from the same reaction.
"This approach allows us to quickly and selectively create complex and useful molecules, giving us more options to explore in chemical space," Paradine says.
More information: Dasha Rodina et al, Ligand control of regioselectivity in palladium-catalyzed heteroannulation reactions of 1,3-Dienes, Nature Communications (2024). DOI: 10.1038/s41467-024-49803-y
Journal information: Nature Communications
Provided by University of Rochester