This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New method reveals secrets of protein interactions with potential for drug discovery
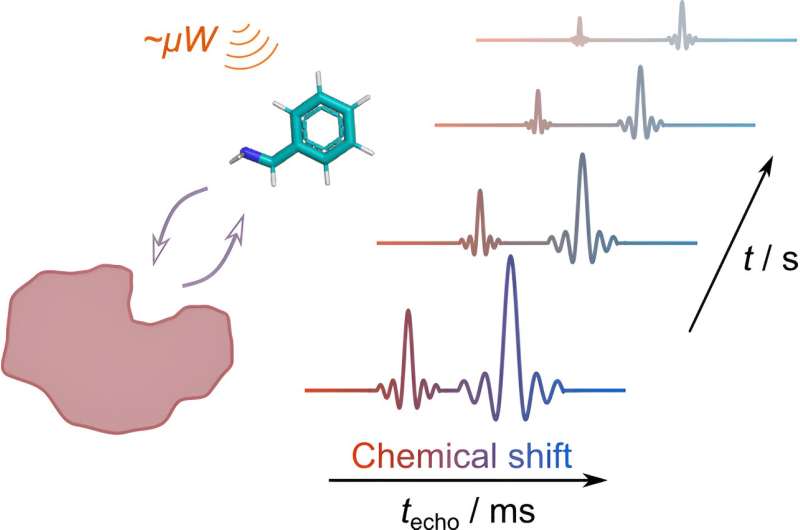
Scientists from the University of Oulu (Finland) and Texas A&M University (U.S.), have developed a new method to study how proteins interact with small ligand molecules, paving the way, for example, for faster and more efficient drug discovery.
This interaction, known as protein–ligand interaction, is crucial for many biological processes, but studying it has traditionally been slow and insensitive. The new method, described in the Journal of the American Chemical Society, combines two advanced techniques to overcome these limitations.
A method has the potential to revolutionize our understanding of protein interactions as part of cells' continuous communication. These interactions and the disruptions that may occur can play a significant role for example in the development of autoimmune diseases and neurodegenerative diseases such as Alzheimer's Disease. For instance, dysfunctional interactions can also lead to aggressive cell growth and cancer.
"The method we have developed could significantly speed up the development of new drugs and help us to understand the mechanisms of many diseases much better," says Dr. Otto Mankinen from the NMR Research Unit, University of Oulu.
Fast and detailed analysis
The first technique, Dissolution Dynamic Nuclear Polarization (d-DNP) hyperpolarization, acts like a signal amplifier, significantly enhancing the signal of the ligand molecule under investigation. Especially when studying low amounts of substances and low abundance nuclei such as carbon-13, hyperpolarization is a crucial tool to make signal observable.
The second technique, Ultrafast NMR, allows the use of hyperpolarization in measurement of multidimensional NMR data. Conventionally measured multidimensional NMR measurements require multiple repetitions to collect full data.
In the ultrafast approach, one of the dimensions is encoded along the sample volume in layers, with a method called spatial encoding. After the encoding, the information is read with the principles of Magnetic Resonance Imaging (MRI). In this case the NMR spectrum was spatially encoded and then the attenuation of signal in time was monitored for multiple spectral peaks.
By combining these techniques, researchers can now obtain detailed information about protein–ligand binding in a single experiment for multiple ligand signals. Conventional approach is limited to a single signal per measurement. This opens doors for more efficient drug discovery by allowing scientists to better understand how potential drug molecules interact with their protein targets.
More information: Chang Qi et al, Measuring Protein–Ligand Binding by Hyperpolarized Ultrafast NMR, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.3c14359
Journal information: Journal of the American Chemical Society
Provided by University of Oulu