This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers develop light-activated protein superglue for fast and precise control of cells and tissues
Researchers at Tampere University have been involved in an international study to develop new tools for the light-activated control of cells. These tools are especially welcome for understanding processes where a fast initial signal leads to long-term changes in cell or tissue function. The modular Lego brick-like structure makes the tools widely applicable in the study of diverse cellular functions.
Controlling biological functions with light has opened new opportunities in a variety of fields, most notably in neurosciences. Light allows tightly controlled activation in a specific location and enables control at different scales, ranging from individual cells to whole organisms. On the molecular level, optical control is often achieved through modified proteins reacting to a specific wavelength of light.
However, with the current tools, the effects are slow to appear, and sustained effects require continuous light activation. This both limits the applicability of these methods in the control of fast processes and leads to unwanted toxicity in the studied cells or organisms.
Together with research groups from the University of Cambridge and University of Pittsburgh, researchers at Tampere University in Finland explored ways to overcome the limitations of existing tools for the light-control of cells. Building on their previous expertise on proteins that form irreversible bonds, the team aimed at achieving an ambitious goal: the fast and cell-friendly control of irreversible protein binding.
The resulting research article, "Visible Light-Induced Specific Protein Reaction Delineates Early Stages of Cell Adhesion," has been published in the Journal of American Chemical Society.
As a starting point, the team used their previously developed "protein superglue," a SpyTag003/SpyCatcher003 peptide/protein pair exhibiting an extremely fast irreversible binding. Based on an engineered Streptococcus pyogenes protein, the SpyTag003/SpyCatcher003 peptide/protein pair allows a Lego brick-like modular assembly of complex protein structures.
To achieve their goal—the optical control of protein superglue—the team had to reach beyond the 20 amino acids constituting human proteins. Using a modified protein synthesis machinery from archaebacteria, the team incorporated a light-reactive unnatural amino acid into the SpyCatcher003 protein. The unnatural amino acid was strategically placed to block the peptide/protein pairing, until its activation by exposure to light.
"A short pulse of light was enough to trigger the rapid and efficient formation of the irreversible peptide/protein complex both in the test tube and in living cells," says Professor Mark Howarth, about the performance of the photoactivated SpyCatcher003. "Importantly, the activation only took place with specific wavelengths of light, making it possible to combine protein control with live-cell fluorescence microscopy."
Having validated their approach for the optical control of an irreversible protein coupling, the team was eager to use the system to answer fundamental questions in cell biology. Human cells attach to the surrounding tissue through cell adhesions; large protein complexes consisting of hundreds of different proteins. Constantly reacting to stimuli arising both inside and outside the cell, cell-matrix adhesions are extremely dynamic.
"Their dynamic structure and vast complexity make cell adhesions difficult to study. The details of how cell-matrix adhesions initially form and how they react to different stimuli have remained largely unknown," says Professor Vesa Hytönen, who has studied the regulation of cell adhesion for years.
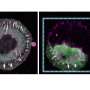
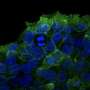
The team split a central adhesion protein, talin, into two halves and explored the use of their newly developed tools for the light-activation of talin protein inside living cells.
"We got very excited when we first realized how well the system worked in controlling complex cellular processes, such as the formation of adhesion and cell spreading. After activating the talin protein with a short pulse of light, we observed an immediate cell response," says Postdoctoral Research Fellow Rolle Rahikainen, the lead author of the research article.
This tight control over adhesion formation allowed the team to explore the earliest events in the formation of cell adhesions. By tracking the timing of protein recruitment into the adhesion complex, the team was able to determine a timeline of events in adhesion complex formation. The findings demonstrated the potential of the light-activated protein superglue for studying complex cellular processes. The results also pave the way for the comprehensive understanding of the complex structure and function of adhesion.
The novel tools for fast and irreversible protein conjugation push the boundaries of what can be done with optical cell control. Fast and irreversible protein conjugation is especially valuable in processes where a short initial signal leads to long-term changes in cell or tissue function. The examples of such processes include the regulation of gene expression during stem cell differentiation and the activation of immune cells in viral infections. Importantly, the modular structure of the novel tools makes them widely applicable in controlling a wide variety of cellular processes.
More information: Rolle Rahikainen et al, Visible Light-Induced Specific Protein Reaction Delineates Early Stages of Cell Adhesion, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.3c07827
Journal information: Journal of the American Chemical Society
Provided by Tampere University of Technology