This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
A novel mechanism by which cells adhere more strongly to their surrounding matrix in response to stress
The DNA molecules in our cells can be damaged by various extrinsic and intrinsic factors called genotoxic stressors; persistent and unchecked damage can lead to developing diseases like cancer. Fortunately, our cells don't sit idly by and let this happen.
In a recent article published in Cell Death & Disease, a team led by researchers at Tokyo Medical and Dental University (TMDU) describe a novel cellular response to genotoxic stress: a newly identified mechanism involving focal adhesions. Focal adhesions are protein assemblies that allow cells to adhere to and interact with their surrounding matrix.
DNA repair pathways and other responses to genotoxic stress have been well described, but the importance of cell-matrix adhesion, and the focal adhesions responsible for this, is being increasingly appreciated. Some studies have shown that genotoxic stress causes loss of focal adhesions and cell detachment from the matrix, while others reported stronger adhesion.
Therefore, the TMDU group became interested in the specific molecular details regarding focal adhesion modification following genotoxic stress and how this process can impact diseases like cancer. They focused on two critical focal adhesion proteins: focal adhesion kinase (FAK) and FAK-related non-kinase (FRNK).
"FAK is one of the most important proteins for cell adhesion to the extracellular matrix, while less is known about FRNK," says Masatsune Tsujioka, lead author of the study. "We are interested in examining this because the modification of cell adhesion can enhance the spread of cancer by allowing cell movement from the tumor."
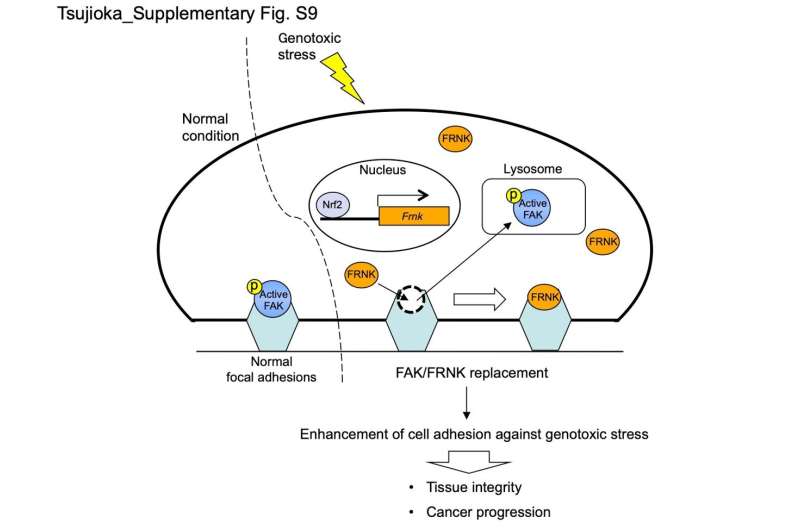
After treating cells with a DNA-damaging agent, the team observed that FRNK expression increases in response to genotoxic stress, and also found that FRNK replaced FAK in focal adhesions.
"Our data suggest that the remodeling of focal adhesions occurs in cells responding to genotoxic stress," explains Shigeomi Shimizu, senior author of the article. "This mechanism involves one protein replacing another within the overall assembly, which makes it unique because remodeling typically involves simple addition or release of the components in focal adhesions."
Additional experiments demonstrated that FAK/FRNK replacement strengthened cell-matrix adhesion, with FRNK stabilizing focal adhesions, leading to firm cell attachment. Interestingly, genotoxic stress severely affected the stomachs of mice that had the gene encoding FRNK knocked out, indicating the significant protective role of FRNK against genotoxic stress.
"Our findings also have disease relevance, as cancer dissemination and progression were inhibited in a mouse cancer model with FRNK depletion," says Tsujioka. "We also analyzed various human colon cancer samples and observed FRNK expression mainly in samples where the cancer had spread rather than in the main tumors. This shows how this mechanism has different biological effects depending on whether it is in a normal tissue or tumor context."
Overall, the team's impactful data demonstrate how a novel focal adhesion remodeling process can strengthen cell adhesion in response to genotoxic stress. While this mechanism helps protect normal tissues, it can also support cancer progression and spread.
More information: Masatsune Tsujioka et al, Identification of a novel type of focal adhesion remodelling via FAK/FRNK replacement, and its contribution to cancer progression, Cell Death & Disease (2023). DOI: 10.1038/s41419-023-05774-4
Provided by Tokyo Medical and Dental University