Ultrastructure of focal adhesion scaffold unveiled in human pluripotent stem cells
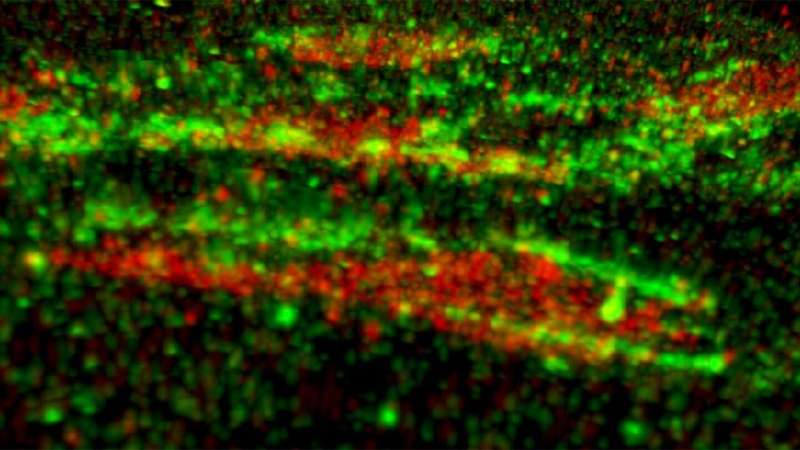
Focal adhesions are known as signaling platforms broadcasting the information of the biochemical and physical qualities of the extracellular matrix into intracellular signaling cascades. However, focal adhesions remain unstudied in the context of human pluripotent stem cells. The research group led by Academy Professor Johanna Ivaska from the Turku Bioscience Centre at the University of Turku unveiled the ultrastructure of focal adhesion scaffold using state-of-the-art super-resolution microscopy in collaboration with the world-renowned Howard Hughes Medical Institute's Janelia Research Campus.
Human pluripotent stem cells hold great promise for regenerative medicine. These cells can differentiate to virtually all adult cell types and proliferate endlessly. So far, the implementation of therapies derived from pluripotent stem cells into the clinics is dragging. This might be due to the lack of understanding the fundamental cell biological properties, like cell-extracellular matrix interaction, governs pluripotency. The ultrastructural peculiarities revealed by this study might provide novel insights into how human pluripotent stem cells co-operate with their surroundings.
"Our results reveal that abrogation of focal adhesion structure leads to speeded up exit from pluripotent state. Also, our three-dimensional super-resolution imaging exposed multiple previously undescribed features in pluripotent cell focal adhesion scaffold. These results imply that focal adhesions could be important in the context of pluripotency," says Doctoral Candidate Aki Stubb, who is the first author of the publication.
Results Might Help Control Cell Differentiation
Conducted at HHMI's Janelia Research Campus in Virginia in the U.S. by Professor Ivaska's team, the interferometric photoactivated localization microscopy (iPALM) experiments provided detailed information of the ultrastructure of the focal adhesions in human pluripotent stem cells. iPALM is capable of achieving sub-20-nm spatial resolution and is a perfect tool for investigating protein-rich cellular structures near to the plasma membrane.
Previous studies have revealed that the focal adhesion scaffold is organized into functional layers in somatic cells. In human pluripotent stem cells, conserved functional layers could also be detected. However, the key mechanosensitive proteins Talin1 and Vinculin showcased unusual nanoscale positions. Also, integrins, the cell surface receptors mediating the cell-extracellular matrix adhesion, displayed spatial subtype-specific compartmentalization.
"We do not know the reason or the mechanism which governs the abnormal nanoscale architecture of the pluripotent stem cell adhesions. Still, our results imply that the unique structure might be a feature of pluripotent cells. We are interested in the molecular mechanisms connecting the focal adhesions to transcriptional regulation of pluripotency," says Professor Ivaska.
The next logical step is to investigate if the modification of the focal adhesion structures could guide the cells to differentiate to specific lineages more efficiently.
The study was published in Nature Communications.
More information: Aki Stubb et al. Superresolution architecture of cornerstone focal adhesions in human pluripotent stem cells, Nature Communications (2019). DOI: 10.1038/s41467-019-12611-w
Journal information: Nature Communications
Provided by University of Turku