This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Team develops new drug discovery platform
A highly versatile family of receptors found on the surface of our cells plays a massive role in the multibillion-dollar field of drug discovery. Called G protein-coupled receptors, or GPCRs, they also facilitate a staggering range of human physiological processes, from smell and vision to inflammation and mood regulation.
To provide some sense of the prominent part these tiny membrane proteins play in drug development, consider this: GPCR-targeted drugs account for nearly 30% of the global therapeutic drug market share. And they still hold significant untapped therapeutic potential.
Now, Dr. Patrick Giguère's lab at the uOttawa Faculty of Medicine has developed a new drug discovery platform to fill that gap.
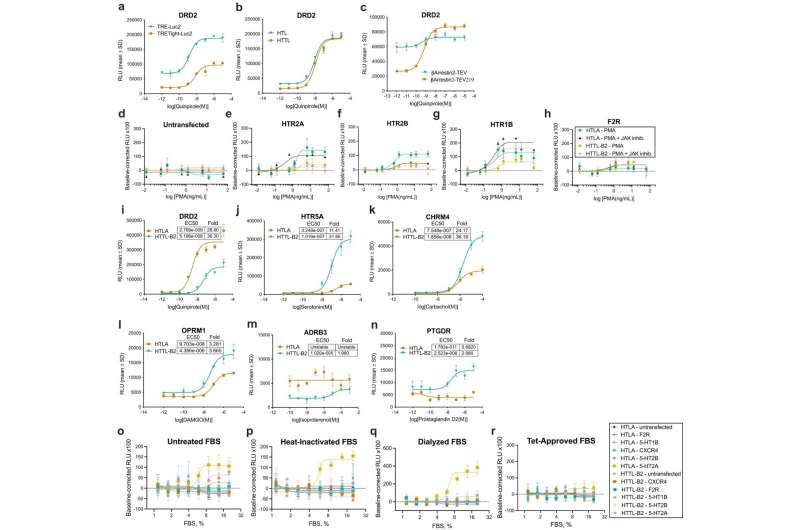
Dubbed "Tango-Trio," the comprehensive screening platform and cellular interrogation tool has the potential to facilitate novel drug discovery targeting a slew of human diseases. The work was published in Nature Communications.
Evolved from an existing platform called PRESTO Tango, the researchers believe that Tango-Trio could facilitate the development of new GPCR-acting drugs and help spur "de-orphanization" efforts. In molecular pharmacology, the term "orphan" is used to indicate that a GPCR is without a known drug or ligand, a molecule that binds to a receiving protein.
"Our platform is one of the most powerful tools for the identification of a new modulator of a specific GPCR target, or conversely the identification of the target for orphan therapy," says Dr. Giguère, an associate professor in the Faculty's Department of Biochemistry, Microbiology and Immunology.
The new platform's knack for targeting orphan receptors is important because it can potentially identify drugs for those orphans whose lack of identified ligands presents a significant challenge to studying them in the first place. Once a small molecule modulator is identified, the GPCR target can be further investigated or validated as a possible therapeutic target.
"Finding a small molecule modulator is thus the key step to study and develop a potential therapeutic," says Manel Zeghal, the study's first author. She's a Ph.D. student in Dr. Giguère's lab who conducted the cell-based experiments.
Zeghal says many potential therapeutic targets have been identified in the uOttawa team's studies.
One of Tango-Trio's strongest capabilities is its ability to detect GPCR signaling referred to as "constitutive activity." It's one of the only platforms of its kind that can pick up this signaling. The constitutive activity of GPCRs is important for numerous reasons, including regulating cellular and physiological processes that are independent of ligand binding. It has also been implicated in various disease states.
Initially, the project was expected to take a couple of years to complete. But the ambitious work took twice that long, underlining the rigor of the research and illustrating how there's no other platform of similar caliber presently available on the market.
"There were many checkpoints throughout the process, where we had to take a step back and rethink the approach. When working with living organisms, such as cells, every step takes time. But in the end, we wanted to create a versatile, open-source resource platform that can be used in any lab around the world, so the time invested was worthwhile," Dr. Giguère says.
The development of this new "open source" resource means that it is accessible to academic researchers across the globe working on exploiting untapped GCPRs, particularly in genetic and immune system disorders.
What are the next steps for his uOttawa lab as his team explores questions suggested by this exciting work? Numerous studies have directly evolved from this new platform so far, according to Dr. Giguère, and various future collaborations are potentially in the mix.
"Indeed, we have been contacted by different researchers to test their drugs or extracts to identify the potential GPCR target and characterize how the drug signals with respect to beta-arrestins and receptor internalization," he says.
Additionally, the Giguere lab is strongly committed to the development of safer painkillers that harness the opioid system without the liabilities associated with actual opiates such as fentanyl or morphine leading the opioid crisis. "We use our platform to test the selectivity of newly developed drugs, but also to better understand the mechanism of action of known opiates."
More information: Manel Zeghal et al, Profiling of basal and ligand-dependent GPCR activities by means of a polyvalent cell-based high-throughput platform, Nature Communications (2023). DOI: 10.1038/s41467-023-39132-x
Journal information: Nature Communications
Provided by University of Ottawa