This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Protein engineers navigate toward more targeted therapeutics
More than a third of FDA-approved drugs work by targeting a G protein-coupled receptor, or GPCR. The human body has more than 800 types of GPCRs that provide cells with information about the external environment to calibrate responses. Drugs that either block or activate GPCRs are used to treat a wide range of diseases including hypertension, pain and inflammation. Most drugs bind to the outside of the receptor, but this can result in adverse side effects since receptors often resemble one another.
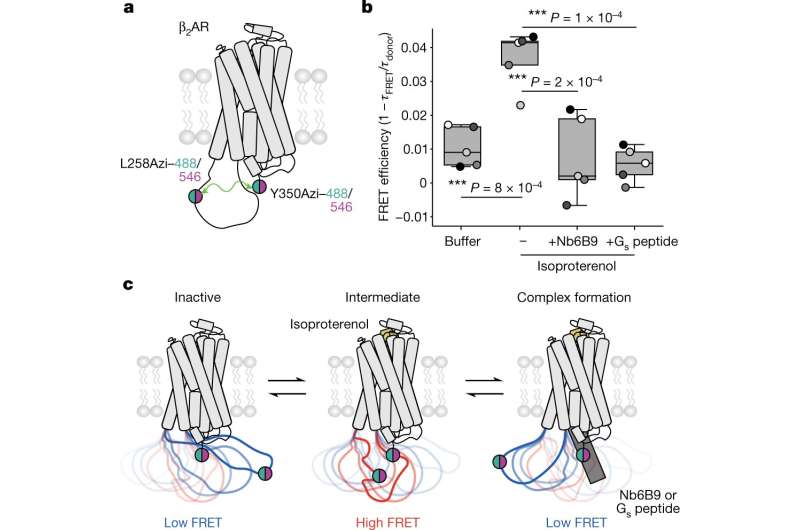
In a new study published in Nature, Sivaraj Sivaramakrishnan, a professor in the University of Minnesota College of Biological Sciences, along with graduate student Fred Sadler and co-authors Michael Ritt and Yatharth Sharma, uncovered the role of the third intracellular loop in the GPCR's signaling mechanism, suggesting the possibility of a more targeted approach to drug discovery and a paradigm shift for new therapeutics.
"Typical GPCR drugs act as on or off switches for cellular signaling outcomes," said Sivaramakrishnan. "Drugs that leverage the loop effectively can act as signaling dimmer switches to more precisely control drug responses."
The authors developed new biochemical and biophysical tools, combined with computational measurements by collaborators Ning Ma and Nagarajan Vaidehi at the City of Hope Cancer Center. They tracked how the third intracellular loop changes in shape, or conformation, through the receptor signaling process. In a breakthrough for the field, their data show that the loop acts as a kind of gate to ensure that receptors activate the correct type of G protein signaling at the right intensity.
"A key advantage of this loop is that it is highly unique, even among closely related receptors, making it an outstanding drug target," said Sadler. "Developing drugs through this newly discovered mechanism would allow for far more targeted therapeutics."
More information: Fredrik Sadler et al, Autoregulation of GPCR signalling through the third intracellular loop, Nature (2023). DOI: 10.1038/s41586-023-05789-z
Journal information: Nature
Provided by University of Minnesota