This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers map fibrillization process to reveal mechanisms of amyloid polymorphism
Amyloids are protein aggregates that can form in the body, sometimes leading to diseases such as Alzheimer's. These fibrils can adopt multiple shapes, known as "polymorphs," which complicate our understanding of their role in health and disease. The ability to morph into different structures makes them both fascinating and challenging to study.
Despite their importance, the exact mechanisms behind amyloid polymorphism have remained elusive. Traditionally, scientists recognized two main types of amyloid structures: twisted ribbons and helical fibrils. However, this view is expanding as new research reveals even more complexity.
Amyloid fibrils can occur with a great number of different molecules, such as insulin. Studying insulin fibrils, scientists at EPFL and ETH Zurich have now made a significant breakthrough in understanding amyloid polymorphism.
Their work shows that insulin amyloid fibrils can twist and bend into complex, "mixed-curvature" shapes by intertwining smaller protein strands in a layered manner, known as "hierarchical protofilament intertwining."
The research was led by the groups of Giovanni Dietler and Henning Stahlberg at EPFL, and Raffaele Mezzenga at ETH Zurich, and is now published in Advanced Science.
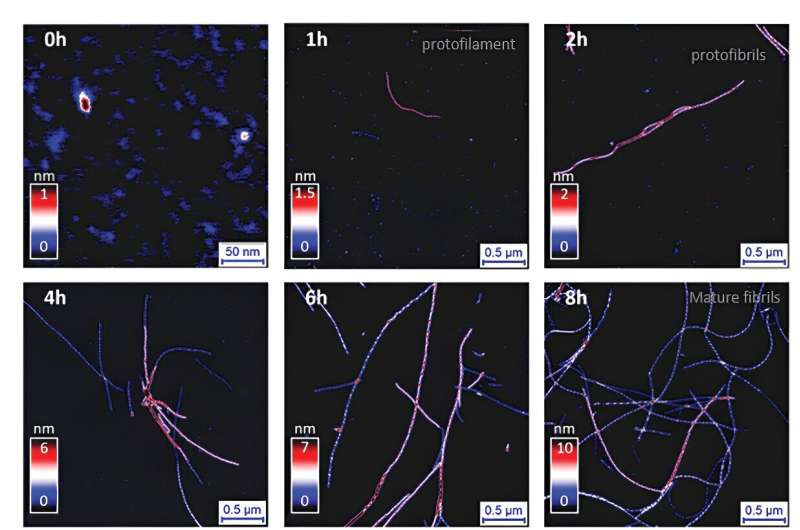
To explore the formation of insulin amyloid fibrils, the researchers used atomic force microscopy (AFM), a high-resolution imaging technique that uses a tiny mechanical probe to scan and map the surface of a sample at the nanometer scale. With AFM, they monitored the fibrillization process, observing its evolution through the typical stages of nucleation, growth, and saturation.
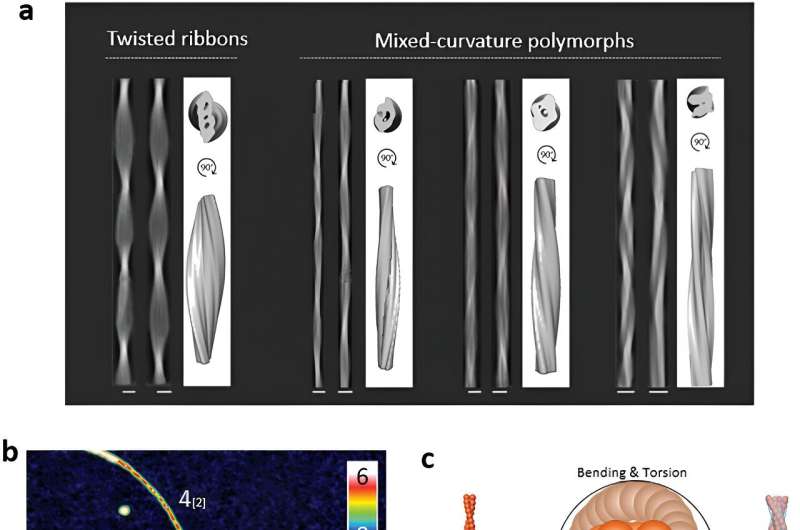
Through detailed analysis, they categorized the fibril morphologies based on their height, crossover pitch, and amplitude. This approach allowed them to identify a wide array of multistranded fibril structures, revealing a complex landscape of amyloid polymorphism.
The study uncovered that insulin amyloid fibrils can form mixed-curvature polymorphs by intertwining protofilaments and protofibrils—the smaller units of fibrils. Protofilaments are basic structural units, made of protein molecules. When they align and twist together, they form protofibrils, which are intermediate structures that eventually combine to form mature fibrils.
This "hierarchical protofilament intertwining" involves both twisting and bending, creating complex structures with unique features. In fact, the researchers found that these mixed-curvature polymorphs are more common than previously thought, especially during extended incubation periods.
They also found that most insulin protofilaments and fibrils exhibit a left-handed twist, a characteristic that persists as the fibrils mature, which adds the layer of chirality (left or right "handedness") to our understanding to amyloid polymorphism.
The study has significant implications for treating amyloid-related diseases. By shedding light on the intricate process of protofilament intertwining, it provides new targets for therapeutic intervention.
Beyond medicine and drug development, the findings could also lead to the development of novel materials based on amyloid fibrils, with potential applications in biotechnology and materials science.
More information: Jiangtao Zhou et al, Hierarchical Protofilament Intertwining Rules the Formation of Mixed‐Curvature Amyloid Polymorphs, Advanced Science (2024). DOI: 10.1002/advs.202402740
Journal information: Advanced Science
Provided by Ecole Polytechnique Federale de Lausanne