Researchers determine three-dimensional structure of PAPP-A
Danish researchers have determined the three-dimensional structure of the proteolytic enzyme PAPP-A. The results may allow us to better understand the basic biology that regulates linear growth of vertebrates. The same regulatory mechanisms are also involved in several age-related diseases, and thus, the research is an important step towards the development of novel types of drugs.
The growth factor IGF plays a key role in human growth. In the absence of IGF signaling, we become dwarfs. Later in life, IGF is involved in age-related diseases, such as cancer and cardiovascular disease. In both cases, IGF must be converted from an inactive to an active form. This is what PAPP-A is able to do.
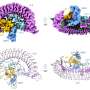
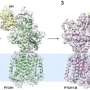
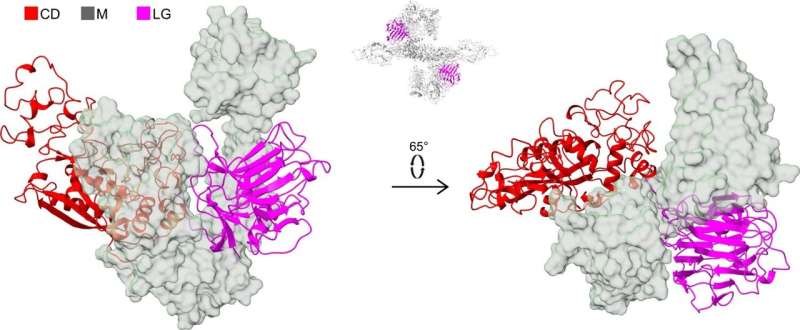
"Seven years ago we discovered that the protein STC2 blocks the activity of PAPP-A, thus indirectly inhibiting the activity of the IGF growth factor. To block the activity, STC2 must form a complex with PAPP-A. We have studied this complex, and we now know its three-dimensional structure," Professor Claus Oxvig explains.
"It is fascinating to see what a molecule, we know biochemically very well, actually looks like. PAPP-A is heart-shaped with an inner 'chamber'. But from a research point of view, the shape is not the most interesting feature. Rather, it is the interactions between the different elements of the molecule."
There are still many unanswered questions about the molecular mechanisms, which regulate how much IGF is converted into the active form. It is likely that complex formation between PAPP-A and STC2 is highly regulated. Such a hypothesis is supported by earlier findings showing that natural human variants of STC2, in which just a single amino acid is substituted, form the complex with PAPP-A slightly slower. The consequence of this is that slightly more IGF can be activated by PAPP-A, resulting in an increase in height of up to 2.1 cm.
The first-author of the publication reporting the PAPP-A·STC2 structure, graduate student Sara Dam Kobberø, has used cryo-electron microscopy (cryo-EM) to determine the structure of the large protein complex. The Danish National Cryo-EM Research Infrastructure (EMBION, AU) has allowed this study, which has also involved participants from the University of Copenhagen.
The research was published in Nature Communications.
More information: Sara Dam Kobberø et al, Structure of the proteolytic enzyme PAPP-A with the endogenous inhibitor stanniocalcin-2 reveals its inhibitory mechanism, Nature Communications (2022). DOI: 10.1038/s41467-022-33698-8
Journal information: Nature Communications
Provided by Aarhus University