Safe, sustainable photo-on-demand synthesis of polypeptide precursors
In nature, there are animals that make fibers that are strong and elastic—for example, the thread that spiders produce to make webs. These fibers have a polypeptide structure and serve as inspiration for research into the development of functional materials.
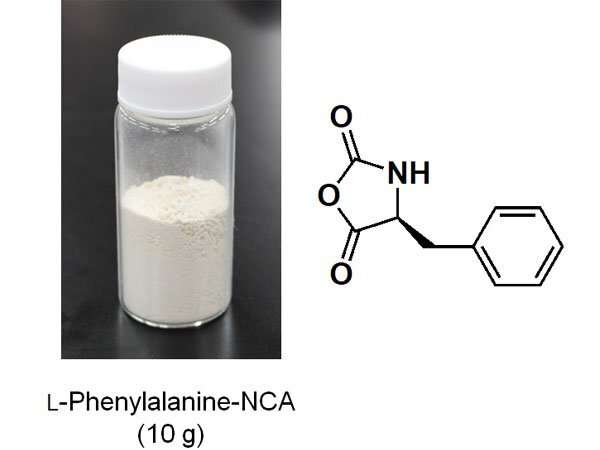
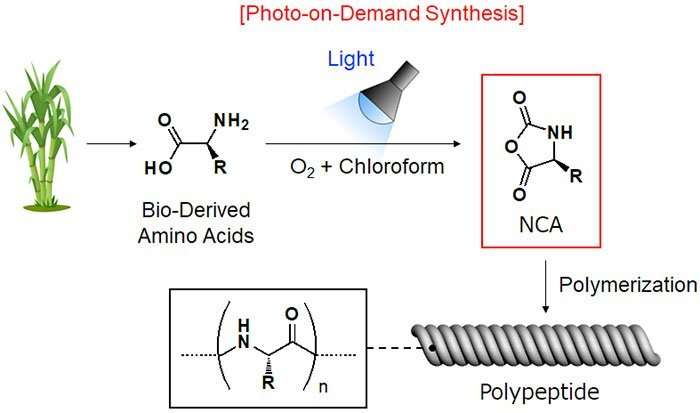
Alpha (α)-amino acid N-carboxyanhydrides (NCAs) are precursors for artificial polypeptides. However, this compound decomposes easily, making it difficult to obtain commercially. Therefore, it is necessary to synthesize the right quantity of α-amino acid NCAs at the location and time that they are required.
NCAs are usually synthesized from plant-derived amino acids and phosgene. However, phosgene is extremely toxic and dangerous to use, leading to growing demand for new chemical compounds and reactions that can be substituted for it. Using the photo-on-demand phosgenation method that they previously developed, Associate Professor TSUDA Akihiko's research group at Kobe University's Graduate School of Science has succeeded in synthesizing NCA in a safe, inexpensive and simple manner from chloroform (a common organic solvent) and amino acid.
The academic paper was published online in ACS Omega on October 19, 2022.
Phosgene (COCl2) is used as precursor for polymers and as a pharmaceutical intermediate. The global phosgene market continues to grow by several percent each year, with around 8 to 9 million tons produced annually. However, phosgene is extremely toxic. For safety reasons, research and development is being conducted to find alternatives.
In a world-first discovery, Associate Professor Tsuda's research group irradiated chloroform with ultraviolet light, which caused it to react with oxygen and generate high yields of phosgene (patent no. 5900920). In order to do this in an even safer and easier manner, the research group found a way that the phosgene-generating reactions could be instantly performed. They first dissolved the reactants and catalysts in chloroform, and generated phosgene by irradiating the solution with light (patent no. 6057449). In this way, phosgene-based organic synthesis can be carried out as if phosgene wasn't used.
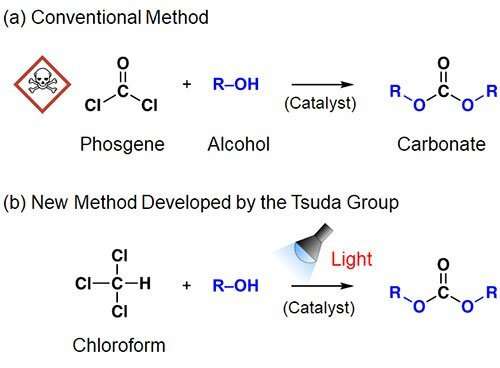
The research group has named their discovery "photo on demand organic synthesis method" and have successfully used it to synthesize numerous useful organic chemicals and polymers. For example, they successfully synthesized large quantities of chloroformate and carbonate in a safe, inexpensive and simple manner merely by irradiating a mixed solution of chloroform and alcohol (with a base added as needed) with light.
These highly original reactions developed at Kobe University have been improved through cooperation with domestic chemical companies, and the eventual aim of this research is practical implementation. Further applied research is being conducted, as well the development of functional polyurethane using this synthesis method.
The photo-on-demand organic synthesis method is highly safe and economical, in addition to having a low impact on the environment. Consequently, it has garnered attention from both industry and academia as a sustainable chemical synthesis method.
Research methodology
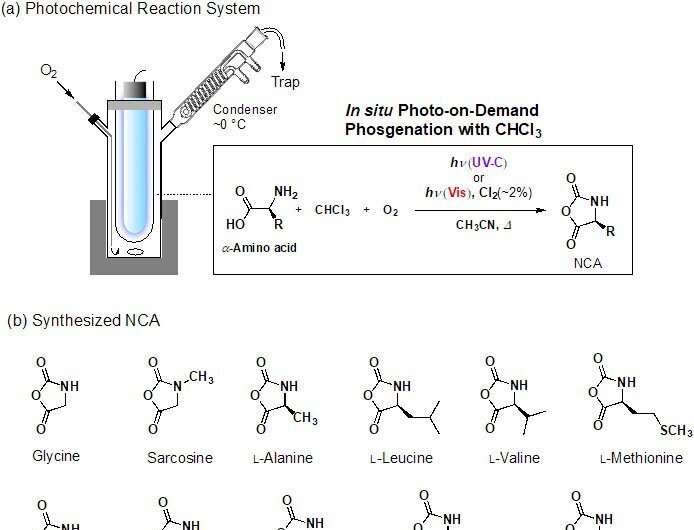
In this research, α-amino acid N-Carboxyanhydrides (NCAs) were successfully synthesized from the raw materials chloroform and α-amino acid using the photo-on-demand method (Figure 2). NCA is a polypeptide precursor. Although α-amino acid dissolves easily in water, it doesn't in chloroform. This meant that the research group had not been able to synthesize NCA using the previous photo-on-demand method. However, they discovered that by adding acetonitrile (CH3CN), which can be mixed with water and chloroform as a solvent, a high yield (around 91%) of NCA could be produced. The reaction was not expected to proceed normally because acetonitrile absorbs the light, hindering the photo oxidation of the chloroform.
Surprisingly, the researchers discovered that the reaction occurred in spite of this hindrance, leading to this study's successful results. Apart from the raw material (amino acid) degraded by the light, this photoreaction can also be used to produce NCAs that are normally synthesized using the phosgene method. So far, the research group has successfully synthesized 11 types of NCA using this photoreaction.
A detailed breakdown of the synthesis method is as follows. Firstly, α-amino acid is suspended in a mixed solution of chloroform and acetonitrile. This is then photo-irradiated for two to three hours at 70°C. After the lamp is switched off, NCA is generated by heating and stirring the solution for around one hour. This product can be extracted and refined to obtain highly pure NCA. The photo-oxidation of the chloroform is promoted by a radical chain reaction that is initiated by the light cleaving C-Cl bonds. Therefore, synthesis can be achieved on a scale of up to 10 grams merely by increasing the size of the reaction container and keeping the light source the same. It is hoped that by further scaling up this method, it can be used in a wide range of fields ranging from academia to chemical industries.
Further developments
The new photo-on-demand NCA synthesis method developed through this research enables large quantities of NCAs (which are polypeptide precursors) to be synthesized in a safe, inexpensive and easy manner. This easy obtainment of NCAs will spur on the research and development of artificial polypeptides, which will hopefully lead to the creation of new materials, such as novel functional polypeptide fibers that will outperform natural fibers produced by animals (Figure 3). In addition, it is expected that synthesizing these new polypeptide fibers using plant-derived amino acid as a starter will enable the development of next-generation biomaterials that meet the needs of the times.
The Tsuda group's goal is for the photo-on-demand NCA synthesis method to be industrially implemented. To this end, they are offering patent licenses and guidance on how to utilize these techniques to interested companies, in addition to continuing their research and development efforts. They hope to develop this research even further by collaborating with industries.
More information: Toshiyuki Sugimoto et al, Photo-On-Demand Synthesis of α-Amino Acid N-Carboxyanhydrides with Chloroform, ACS Omega (2022). DOI: 10.1021/acsomega.2c05299
Journal information: ACS Omega
Provided by Kobe University