New eco-friendly synthesis method uses alumina as a recyclable catalyst

An international research collaboration between Kobe University and Inner Mongolia Medical University has developed a simple, low cost and comparatively environmentally friendly method of synthesizing diphenylmethanol derivatives using alumina from China. Diphenylmethanol derivatives are used as raw materials in the manufacture of perfumes and pharmaceuticals, among others.
The researchers discovered that alumina can be repeatedly reused for this reaction if it is washed with water and dried between usages. This recycling reduces both the need for more alumina and the amount of waste produced, lowering synthesis costs and the impact on the environment. As global environmental awareness continues to increase, the researchers hope that this new method of chemical synthesis will contribute towards realizing a carbon neutral society and achieving the SDGs.
This discovery was made by an international research group, which included Associate Professor Tsuda Akihiko of Kobe University Graduate School of Science (who is also a visiting professor at Inner Mongolia Medical University) and researchers from Inner Mongolia Medical University, including Professor Chaolu Eerdun (who obtained their Ph.D. from Kobe University's Graduate School of Science) and Lecturer Liang Fengying.
A patent application for this method was filed in China in April 2021, with a priority claim application made in September of the same year. Subsequently, the results of this research were published online in the academic journal ChemistryOpen on May 18, 2022.
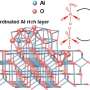
Alumina (Al2O3) is an aluminum oxide mainly used as a raw material for the production of aluminum (Figure 1). However, it is also utilized as a catalyst in the field of organic synthetic chemistry. It is mainly used for reactions that require harsh conditions (such as high temperature or high pressure). However, alumina is not a commonly-used catalyst for various reasons, one being that it can only be used for a small range of chemical reactions. Alumina is also used to adsorb impurities in the organic synthesis field and as a stationary phase substance in chromatography. However, issues such as its high cost as a raw material and the large amount of non-burnable waste it generates means that there is a trend towards replacing it with substitutes. Under these circumstances, Professor Tsuda led a research group at Inner Mongolia University (China) that succeeded in developing a new, sustainable method of organic synthesis using alumina, which China produces in great quantities.
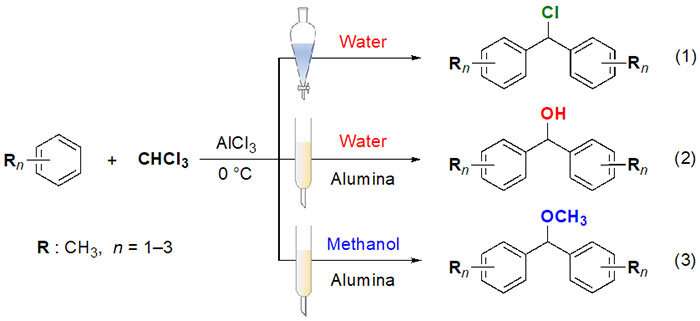
Professor Tsuda and the Inner Mongolia Medical University team discovered a simple, low-cost, environmentally friendly way of synthesizing diphenylmethanol derivatives (which are starting materials in the production of fragrances and pharmaceuticals) by utilizing Chinese alumina as both a catalyst and adsorbent (Figure 2). Using aluminum chloride (AlCl3) as a catalyst, the generic organic solvents toluene, xylene, and trimethylbenzene were reacted with chloroform. If the resulting substance is post-treated with water, then you mainly obtain a chlorination product. However, the researchers discovered that if the same resulting substance is deposited on alumina that contains water, you can obtain diphenylmethanol derivatives. They also found that if the resulting substance is deposited on alumina that contains methanol, a methanol substitute is obtained. It is thought that when the substance is adsorbed onto the alumina, it reacts with the water or alcohol within to produce the respective end product.
Furthermore, the research group found that a highly pure product could be obtained even if it was adsorbed by alumina that had previously been used as a catalyst or was an impure by-product. Using the aforementioned method it is possible to selectively synthesize three different products.
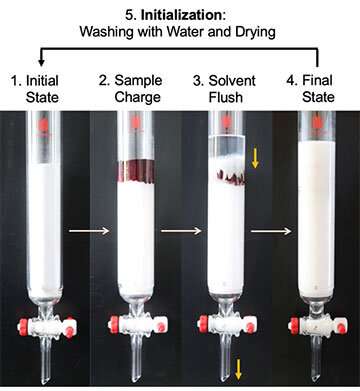
However, commercial alumina is comparatively expensive, which would make it difficult to implement reactions like this that require large amounts on an industrial scale. With this in mind, the researchers tried reusing the alumina after rinsing it with water and allowing it to dry, and discovered that it retained its catalytic and adsorbent properties (Figure 3). This recycling process can be performed repeatedly, which greatly reduces the cost of materials as well as the amount of waste. For the synthesis experiments in the laboratory, the amount of alumina used was in the region of a few grams to tens of grams. It is a safe, high-yield reaction that only takes a short amount of time to complete (a number of hours), therefore this application of alumina on an academic level could be expanded into diverse fields in the chemical industry. It is hoped that it could provide society with a practical and sustainable organic synthesis method.
At 0°C, aluminum chloride (1.1 g, 8 mmol) was added to a mixture of chloroform (30 mL, 0.37 mol) and an aromatic substrate such as p-xylene (1mL, 8mmol), and then stirred for six hours. After this, the resulting sample solution was dropped into a commercially available alumina column (water content ~1 wt%) and subjected to column chromatography with a dry chloroform/ethyl acetate (1:1) eluent. This chromatography revealed that a 94% yield of diphenylmethanol derivatives can be produced using this method. Refinement processes such as recrystallization can be performed as required to obtain a highly pure end product.
As for the mechanism behind this, it is thought that the chloroform and the aromatic substrate undergo an aluminum chloride-mediated Friedel-Crafts reaction. The resulting reactant and aluminum chloride are adsorbed by the alumina and are subsequently hydrolyzed by the water molecules in the alumina, leading to the formation of the end product. After removing the end product from the alumina, the alumina can be recycled by first washing away the adsorbed compounds, salts and solvents remaining in the alumina and then drying it. Consequently, the alumina can be reused as a catalyst for this reaction again and again.
The novel catalytic, adsorbent and recyclable properties of alumina discovered through this research have potential applications to the organic synthesis of compounds other than diphenylmethanol derivatives. The goal is to greatly expand this reaction's range of applications to develop a more general synthesis method that can be used to produce various useful chemicals.
Amidst rising global environmental awareness, it is hoped that the new chemical reaction developed in this study will become a novel method of synthesizing chemical products which will contribute towards recycling efforts, carbon neutrality and the SDGs. It is predicted to bring about fresh innovation in the organic synthesis and organic chemical industries. It is hoped that continued development of this method through the international research collaboration with China, the world's number one producer of alumina, will result in highly practical large-scale implementation.
More information: Guga Suri et al, Direct Syntheses of Diphenylmethanol Derivatives from Substituted Benzenes and CHCl 3 through Friedel‐Crafts Alkylation and Post‐Synthetic Hydrolysis or Alcoholysis Catalyzed by Alumina, ChemistryOpen (2022). DOI: 10.1002/open.202200042
Provided by Kobe University