This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Transforming inexpensive quinolines into complex drug candidates
An innovative synthesis strategy has opened the way to 2D/3D fused frameworks using inexpensive quinolines as feedstock, report scientists from Tokyo Tech. By leveraging a light-sensitive borate intermediate, the scientists could transform quinoline derivatives into a great variety of 2D/3D fused frameworks in a straightforward and cost-effective manner.
Their findings, which are published in Angewandte Chemie International Edition, are expected to enable the synthesis of highly customizable drug candidates.
Quinolines have garnered much attention from chemists wanting to synthesize compounds known as 2D/3D fused frameworks. These complex organic molecules have a lot of medical potential due to their highly customizable structure and functional groups.
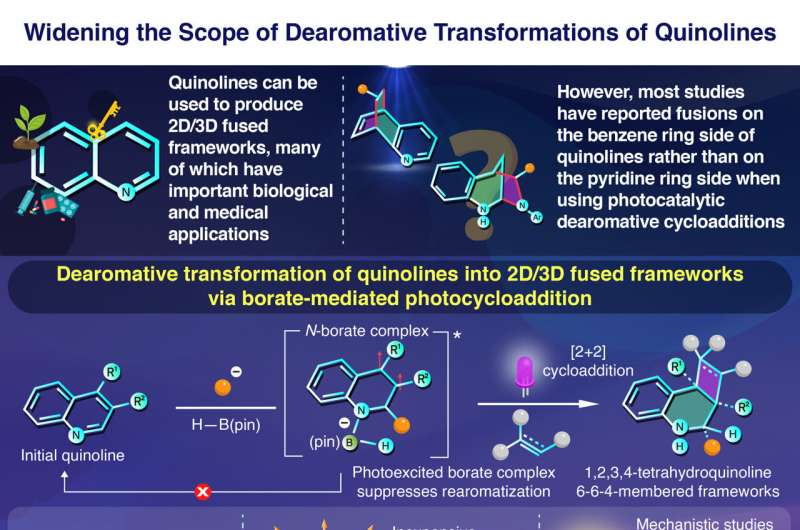
Quinolines make synthesis of such frameworks possible thanks to their unique electronic configuration. They consist of an electron-abundant benzene ring fused to an electron-deficient pyridine ring; these electronically distinct rings can be modified independently by adjusting reaction conditions.
One of the most attractive ways to use quinolines as feedstock for 2D/3D frameworks is through dearomative photocycloadditions. This process involves destabilizing one of the aromatic rings in quinoline, using light and sometimes a catalyst, so that a reactant can 'latch' onto the ring, forming the target compound.
Despite many efforts, most studies have reported photocycloadditions happening on quinoline's benzene ring side, while few have targeted the pyridine side. Thus, the full potential of quinolines remains untapped.
Against this backdrop, a research team from Tokyo Institute of Technology, Japan, led by Assistant Professor Yuki Nagashima, in collaboration with scientists from The University of Tokyo, decided to step up to the challenge. In their latest study they present a convenient methodology to access the pyridine side of quinolines and synthesize diverse 2D/3D frameworks.
The key to their approach lies in the use of a molecule known as pinacolborane, also known as H–B(pin). The researchers discovered that this boron-containing compound was extremely effective at inducing dearomative photocycloaddition almost exclusively on the pyridine side of quinoline. Not only were the yields obtained high but also could a wide variety of quinoline derivatives and reactants be used to obtain all sorts of 2D/3D frameworks.
Seeking to shed light on the mechanisms underlying their newfound strategy, the team conducted a series of experiments and theoretical analyses. They determined that quinoline reacts with an organolithium compound first and then with H–B(pin) to form an intermediate borate complex.
This intermediate step is crucial, as Nagashima comments, "Our detailed mechanistic studies revealed that the photoexcited borate complex both accelerates the cycloaddition and suppresses the rearomatization that usually occurs in conventional photocycloaddition reactions." These effects combined lead to fewer unreacted compounds and undesired aromatics.
It is worth noting that the proposed methodology offers several advantages over competing techniques. For one, it requires less reaction time and steps than classic synthesis routes. Catalysts are not needed either, which reduces costs further. Moreover, multi-substituted starting molecules can be used, which provides access to countless target compounds.
"To our knowledge, these transformations are the first boron-based photocycloadditions and unlock previously elusive organoboron compounds. The present strategy based around an intermediate borate complex should be useful for further functionalization of various types of multi-ringed aromatic hydrocarbons," says Nagashima.
More information: Asuha Shimose et al, Dearomative Construction of 2D/3D Frameworks from Quinolines via Nucleophilic Addition/Borate‐Mediated Photocycloaddition, Angewandte Chemie International Edition (2024). DOI: 10.1002/anie.202403461
Journal information: Angewandte Chemie International Edition
Provided by Tokyo Institute of Technology